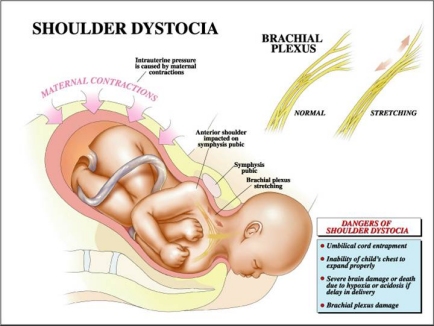
The greatest nightmare an obstetrician is likely to face is shoulder dystocia. At an otherwise normal delivery, just after the baby's head has emerged, the neck suddenly retracts back against the mother's perineum causing the baby's cheeks to puff out. The experienced obstetrician knows at this point that the baby's anterior shoulder is caught on the mother's pubic bone and that if he or she is unable to free up the shoulder within a few minutes the baby will suffer irreversible brain damage or death.
Shoulder dystocia occurs in approximately 0.5%-1.5% of all deliveries. Given that there are roughly 3 million babies born vaginally each year in the United States, this delivery complication will be experienced by roughly 15,000-45,000 women a year. The larger the baby, the more likely it is to occur. However, even with very large babies shoulder dystocia occurs only occasionally and sporadically. Therefore a physician never knows when it will be encountered.
The most common serious complication following a shoulder dystocia delivery is brachial plexus injury. This is when the nerves in a baby's neck--the brachial plexus—are temporarily or permanently damaged. The nerves of the brachial plexus control the function of the arm and hand. Injury to the upper part of the brachial plexus is called Erb palsy while injury to the lower nerves of the plexus is called Klumpke palsy. Both can cause significant, lifelong disability.
Because of the gravity and unexpectedness of shoulder dystocia it has long been a major area of obstetrical concern. Yet despite the hundreds of published studies about shoulder dystocia there are still multiple, important recurrent questions: Is shoulder dystocia predictable? Can it be prevented? Is there anything that can be done when it does occur to deliver the baby without brachial plexus nerve damage? If there is an injury, was it caused by mismanagement on the part of the clinician or was it an inevitable consequence of the shoulder dystocia?
The interest obstetricians have in these questions has been heightened in the last two decades by the increasing influence of medical-legal issues on the practice of medicine. As regards shoulder dystocia, it is frequently the case that when a brachial plexus injury occurs, an obstetrician will be charged with negligence. Such claims are now so frequent that law suits related to shoulder dystocia deliveries constitute the second largest category of indemnity payments in obstetrics, exceeded only by birth asphyxia. In their defense, physicians contend that shoulder dystocia is a totally unpredictable event and that even with perfect management brachial plexus injuries will occur. Where does the truth lie?
This web site represents an attempt to answer this and other questions about shoulder dystocia. By having thoroughly reviewed the published literature on shoulder dystocia and brachial plexus injury from 1965 to the present, it has been possible to frame comprehensive and consistent answers to the major questions that bedevil this area of obstetrics. It is the hope of the author that the information presented here about the cause, preventability, and culpability for shoulder dystocia and brachial plexus injuries will (1) aid in improving the care given to women and their babies and (2) will help to fairly adjudicate responsibility in medical liability cases in which a baby has been injured during a shoulder dystocia delivery.
Imortant new document
A recent report by the American College of Obstetricians and Gynecologist (2014) entitled Neonatal Brachial Plexus Palsy, written by a panel of the leading experts on shoulder dystocia and brachial plexus palsy from across the country, has added much specific information and informed opinion to the discussion of shoulder dystocia and brachial plexus palsy. This document can be ordered at acog.org.
History
The phenomenon of shoulder dystocia has long been recognized. Smellie, one of the earliest physicians specializing in obstetrics, described a situation he encountered in 1730 as follows:
Called to a gentlewoman in labor. The child's head delivered for a long time — but even with horrid pulling from the midwife, the remarkably large shoulder prevented delivery. I have been called by midwives to many cases of this kind, in which the child was frequently lost.
Morris in 1955 gave what is now a classic description of shoulder dystocia:
The delivery of the head with or without forceps may have been quite easy, but more commonly there has been a little difficulty in completing the extension of the head. The hairy scalp slides out with reluctance. When the forehead has appeared it is necessary to press back the perineum to deliver the face. Fat cheeks eventually emerge. A double chin has to be hooked over the posterior vulvar commisure, to which it remains tightly opposed . . .
Time passes. The child's face becomes suffused. It endeavors unsuccessfully to breathe. Abdominal efforts by the mother and by her attendants produce no advance. Gentle head traction is equally unavailing. Usually equanimity forsakes the attendants — they push, they pull. Alarm increases. Eventually, "by greater strength of muscle or by some infernal juggle," the difficulty appears to be overcome, and the shoulder and trunk of a goodly child are delivered. The pallor of its body contrasts with the plum-colored cyanosis of the face, and the small quantity of freshly expelled meconium about the buttocks. It dawns upon the attendants that their anxiety was not ill founded, the baby lies limp and voiceless, and only too often remains so despite all efforts at resuscitation.
Perhaps the most famous case of brachial plexus injury was that involving Prince William of Germany who subsequently became Kaiser Wilhelm II in 1888. It seems that William was in breech position at birth and was manipulated by several physicians and a midwife during delivery. Apparently the baby was not breathing when it emerged, but by "continuous rubbing . . . dousing in a hot bath, and a series of short, sharp slaps on his buttocks" the doctors managed to get the child to breathe.
The third day after delivery the midwife noticed that William's left arm was slack. It was thought that the arm had been "wrenched out of the socket" and some of the muscle tissue torn. Most likely, William suffered a brachial plexus injury. In addition it is suspected that there were several moments of asphyxia which might have caused slight brain damage. It has been postulated that this was the cause of William's later hyperactivity and emotional instability. He may also have suffered slight cerebral palsy. For the rest of his life, William's "withered" left arm was concealed from the public by careful posing for photographs.
What is
shoulder dystocia?
Shoulder dystocia occurs when, after delivery of the fetal head, the baby's anterior shoulder gets stuck behind the mother's pubic bone—or, occasionally, the baby’s posterior shoulder impinges on the mother’s sacrum. If this happens, the remainder of the baby does not follow the head easily out of the vagina as it usually does during vaginal deliveries.
This simple definition of shoulder dystocia, however, glosses over many complexities. For example, should a delivery be categorized as involving shoulder dystocia only when there is some time delay—60 seconds is often suggested in this context—between the delivery of a baby's head and shoulders? Or is shoulder dystocia present any time that a delivering clinician finds that the shoulders cannot be delivered with the normal amount of downward traction on the fetal head? Some have suggested that the definition of true shoulder dystocia requires that the birth attendant had to perform special maneuvers in order to deliver the shoulders.
Exactly how shoulder dystocia is defined is more than just a semantic issue. It sets the parameters for the collection of statistics related to shoulder dystocia, a necessity for research aimed at decreasing shoulder dystocia related injuries. It also determines when a baby's injuries might be attributed to a physician's actions during labor and delivery. For instance, if there was no true shoulder dystocia during a particular birth in which there is a brachial plexus injury, can the physician reasonably be blamed for having applied excessive traction?
click on
image to view larger image

Pelvic
anatomy related to shoulder dystocia
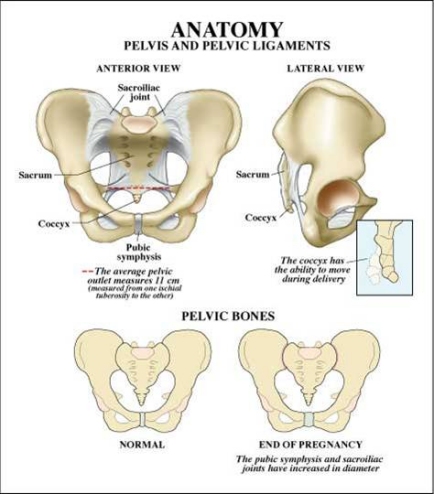
It is necessary to know something about the anatomy of the fetus and the maternal pelvis in order to understand how shoulder dystocia comes about and how it causes the injuries it does.
As the accompanying diagram shows, the maternal pelvis is composed of a series of bones forming a circle protecting the pelvic organs. The front-most bone is the symphysis pubis. It is on this structure that a baby's anterior shoulder gets caught during a delivery complicated by shoulder dystocia. The bone at the back of the maternal pelvis is the sacrum. Because of its shape, it generally serves as a slide over which a baby's posterior shoulder can descend freely during labor and delivery. However sometimes a baby’s posterior shoulder can get caught on its slight projection into the pelvis. The side walls of the maternal pelvis, although very important in determining how smoothly the process of labor will go, usually do not contribute to shoulder dystocia.
click on
image to view larger image
In normal vaginal deliveries the head of the baby, called the "vertex", emerges first. During labor, the soft, mobile bones of the fetal head can overlap and the head as a whole can "mould"—go from perfectly round to more pointed and narrower. This facilitates the fetal head fitting into and through the maternal pelvis. The baby's shoulders, likewise being flexible, usually follow the delivery of the baby's head quickly and easily. But for this to happen, the axis of the fetal shoulders must descend into the maternal pelvis at an angle oblique to the pelvis's anterior-posterior dimension. This position affords the shoulders the most room for their passage. If instead the shoulders line up in a straight front-to-back orientation as they are about to emerge from the mother's pelvis, there will often be insufficient room for them to squeeze through. The back of the mother's pubic bone then forms a shelf upon which the baby's anterior shoulder gets caught. If this happens, the shoulders cannot deliver and a shoulder dystocia results.
As previously mentioned, shoulder dystocia can also occur if the posterior shoulder of a baby gets caught on its mother's sacrum. This is a far less common cause of shoulder dystocia. The sacrum, having only a slight protrusion, is far less likely to impede the descent of the baby's posterior shoulder than is the mother’s pubic bone to block passage of the baby’s anterior shoulder.
As can be readily appreciated, it is the relative sizes of the fetal head, shoulders, and chest compared to the shape and size of the maternal pelvis that determine how smoothly a delivery will go. Usually it is the fetal head that has the largest fetal dimensions. Thus if the head can pass through the maternal pelvis without difficulty, the rest of the baby usually follows easily. However, when the dimensions of the fetal shoulders or chest rival those of its head—such as in an especially large baby or a baby of a mother with diabetes—the chances of a shoulder dystocia occurring are much increased. Since larger babies, whether of diabetic mothers or not, are more likely to "get stuck", much of the work in the field of shoulder dystocia has been targeted at attempting to predict which babies will be larger than normal, especially when their mothers are diabetic.
Except in extraordinary circumstances, once the fetal head and shoulders have been delivered the remainder of the fetal trunk and legs slide out easily. Such extraordinary circumstances preventing easy delivery of the fetal body might occur when:
- A fetus has a large abdominal or lower back tumor,
- The umbilical cord is wrapped tightly around the baby's neck, or
- There is a severe constriction of the uterine muscle —"contraction ring" — trapping the baby in the uterus.
The above applies only to vertex (headfirst) deliveries. Breech deliveries, where the fetal legs and buttocks emerge first from the vagina, can also result in injury to the brachial plexus, producing the sorts of injuries discussed above. However, since vaginal breech deliveries are known to produce a higher rate of neonatal morbidity and even mortality, most breech babies in the United States are now delivered by cesarean section.
Incidence
The incidence of shoulder dystocia is generally reported to be between 0.3 % and 1.5% with scattered reports listing values both higher and lower. The "true" incidence of shoulder dystocia, however, is very much dependent upon how it is defined, how it is reported, and the characteristics of the population being measured. For instance:
The Bulletin on Shoulder Dystocia by the American College of Obstetricians and Gynecologists (ACOG) lists the rate of shoulder dystocia as 1.4% of vaginal births.
The rate of shoulder dystocia in Great Britain reported by the Royal College of Obstetricians and Gynecologists as 0.6%
The rate of shoulder dystocia as reported by various authors is as follows:
Nocon (1993) 1.4
Baskett (1995) 0.6%
McFarland (1996) 0.7%
Bofill (1997) 3.3%
Gherman (1998) 1.4%
Stallings (2001) 1.7%
Foad (2008) 1.51
Chauhan (2014) 1.4
Tsur, in a 2011 study from Israel involving 240,000 deliveries, found that shoulder dystocia complicated 0.2% of all deliveries. Interestingly, in this study the rate of shoulder dystocia was seen to have declined from 0.4% in 1988 to 0.13% in 2009. The author feels that this was most likely due to an increasing rate of cesarean deliveries for suspected macrosomia.
Overland (2014), in one of the largest reports on the subject, reviewed data from 1,914,544 vaginal deliveries. The reported rate of shoulder dystocia in that group was 0.68%.
Parantainen (2014) evaluated 42,964 deliveries in Finland. He reported an incidence of shoulder dystocia of 0.42%.
Hansen, in a 2014 review article evaluating 28 published studies on the incidence of shoulder dystocia involving more than 16 million births, found a composite rate of shoulder dystocia of 0.4%.
The accuracy of reporting is an important variable in shoulder dystocia statistics. Many obstetricians are reluctant to write down in their delivery notes that a shoulder dystocia has occurred for fear that this will be a red flag attracting a malpractice suit should it later turn out that the baby suffered an injury. Some studies have shown that only 25% to 50% of shoulder dystocias — as noted by objective observers in a delivery room — are recorded by the delivering physician (Gonik, 1991).
How one defines shoulder dystocia, of course, affects its reported incidence. Some obstetricians will only report a delivery as involving shoulder dystocia if they had to employ specific maneuvers to deliver the baby's anterior shoulder. Others will record shoulder dystocia if there is any delay in the emergence of the shoulder following delivery of the head. In some cases a physician will only record shoulder dystocia when a fetal injury has occurred.
Finally, the characteristics of the delivery group being measured will affect statistics on shoulder dystocia. A study evaluating the incidence of shoulder dystocia in a population with a larger than average percentage of macrosomic neonates or of diabetic mothers will have a much higher reported incidence of shoulder dystocia than if the population were a general one containing a more representational sample of both small and large babies and the normal percentage of mothers with diabetes.
Several recent studies have shown a lower rate of shoulder dystocia than has been reported in the past. This is despite the fact that on average newborns are getting larger. For instance, the percentage of very large babies (>4000 gms) has gone up 300% between 1970 and 1988 (Johar, 1988). Moreover, the last several decades has seen a marked increase in average maternal weight, average maternal weight gain during pregnancy, and the number of diabetic women having babies. All of these factors should have lead to an increase the incidence of shoulder dystocia.
The likely answer to this paradox is several fold:
1. Physicians are now more aware of estimated fetal weight than in years past and are quicker to confirm this with ultrasound
2. Physicians are more reluctant to allow mothers with suspected macrosomic fetuses to have trials of labor but rather are recommending cesarean section for delivery.
3. Diabetic mothers with suspected macrosomic fetuses are especially being steered towards cesarean section for delivery.
One Step, Two Steps: An Interesting Theory
Iffy, in a 2015 article, claims that there has been an increased rate of shoulder dystocia and brachial plexus injury over the past several decades. He speculates that this increase is due to the advocacy of active management of delivery as proposed in the 1976 edition of Williams Obstetrics, whose major authors were Pritchard and McDonnell. This technique, called by some the “one step” technique, recommends attempting to deliver the infant’s shoulder immediately after the birth of the head. Locatelli (2011) has also discussed this issue.
Iffy notes that in prior editions of the Williams textbook (1961), whose major authors were Eastman and Hillman, it was expected that the shoulders would emerge in the contraction following delivery of the head—the “two-step” technique. Iffy denigrates the obstetrical experience of the authors of the newer edition, Prichard and McDonnell, saying that their expertise lay in cardiology, preeclampsia and basic sciences “rather than extensive experience in hands-on obstetrics.” Iffy also goes on to claim that prior to 2005 British obstetricians never adopted the “one step” policy and therefore had lower rates of brachial plexus injury—but that since they, too, have moved to the “one-step” technique they have seen an increase rate of BPI. Iffy concludes from the above that:
The rise of shoulder dystocia incidents since the introduction of active management suggest that elective use of traction is a major predisposing factor for both arrest of the shoulders and the deriving fetal injuries.”
However there are several aspects of Iffy’s contentions that deserve further scrutiny.
First of all, has there really been an increase in shoulder dystocia and brachial plexus injury—or has it just been reported more often due to medical-legal issues and better record keeping?
Second, Iffy’s arguments do not take into account the increasing percentage of macrosomic babies, the pronounced increase in maternal obesity, and the increasing use of epidural anesthesia as potential reasons for any increases in shoulder dystocia and brachial plexus injuries that might in fact have occurred.
Third, Iffy nowhere provides data to show that there is more traction involved in the “one-step” technique than the “two-step” technique.
Finally, Iffy’s argument only holds if clinician traction is the main cause of brachial plexus injuries when shoulder dystocias do occur. As will be shown below, while this has often been contended, it has never been proven—and there is much evidence against this hypothesis.
Recurrent
shoulder dystocia
The question as to whether or not women who have had a shoulder dystocia in a previous delivery are more likely to have one again in a subsequent delivery is an extremely important one as it will help guide how future deliveries in these women are managed.
It appears from the literature that the risk of recurrent shoulder dystocia is substantial: 10 to 15% (Lewis, 1995; Usta, 2008; Overland, 2009). Kleitman (2016) reported that previous shoulder dystocia was found to be an independent risk factor for recurrent shoulder dystocia with an odds ratio of 6:1. Moreover, women who have had a shoulder dystocia delivery that resulted in injury to the fetus have an even greater risk of having a recurrent shoulder dystocia with fetal injury.
Neonatal injuries following shoulder dystocia
Following shoulder dystocia deliveries, 20% of babies will suffer some sort of injury, either temporary or permanent. The most common of these injuries are damage to the brachial plexus nerves, fractured clavicles, fractured humeri, contusions and lacerations, and birth asphyxia.
Brachial plexus injury
Brachial plexus injury is the classic injury following shoulder dystocia. It was first described by Duchenne in 1872.
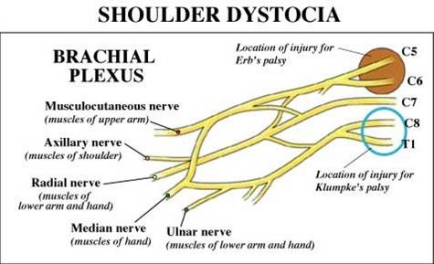
The brachial plexus consists of the nerve roots of spinal cord segments C5, C6, C7, C8, and T1. (See accompanying diagram). These nerve roots form three trunks which divide into anterior and posterior divisions. The upper trunk is made up of nerves from C5 and C6, the middle trunk from undivided fibers of C7, and the lowermost trunk is made up of nerves from C8 and T1.
click on
image to view larger image

There are two major types of brachial plexus injury: Erb palsy and Klumpke palsy.
Erb palsy, the more commonly occurring form, involves injury to the upper trunk of the brachial plexus (nerve roots C5 through C7). This palsy affects the muscles of the upper arm and causes abnormal positioning of the scapula: "winging". The supinator and extensor muscles of the wrist that are controlled by C6 may also be affected. Sensory deficits are usually limited to the distribution of the musculo-cutaneous nerve. Together, these injuries result in a child having a humerus that is pulled in towards the body (adducted) and internally rotated with the forearm extended. Some have described this as the "waiters tip" position.
Klumpke palsy involves lower trunk lesions from nerve roots C7, C8, and T1. In this injury the elbow becomes flexed and the forearm supinated (opened up, palm-upwards) with a characteristic clawlike deformity of the hand. Sensation in the palm of the hand is diminished.
click on
image to view larger image
Patients seen with upper lesions immediately after birth—Erb palsy—have a better prognosis than those with lower brachial plexus injuries—Klumpke palsy. Whereas upwards of 90 to 95% of all Erb palsies totally resolve, only 60% of Klumpke palsies do. Interestingly, those brachial plexus injuries associated with non-shoulder dystocia deliveries persist more often than those occurring following deliveries in which a shoulder dystocia was documented.
Brachial plexus injuries can also produce secondary effects. Muscle imbalances in the hand, arm, and shoulder caused by brachial plexus injuries may result in osseous deformities of the shoulder and elbow and in dislocations of the radial head. The development of the affected arm may be compromised resulting in its being as much as 10 cm shorter than the nonaffected arm.
While a sense of the degree and type of injury can be estimated by physical exam and clinical observation of the baby’s movement limitations, the true extent of brachial plexus injuries and the specific pathophysiology involved can only be definitively determined during surgical exploration of the brachial plexus at the time of a reparative procedure.
It has been traditionally thought that most brachial plexus injuries result from stretching of the nerves of the brachial plexus during delivery. While this likely accounts for many brachial plexus injuries, reports of such injuries following deliveries in which there was no shoulder dystocia (Allen 2005, Lerner 2008, Ouzounian 2012) has led investigators to question whether or not brachial plexus injuries might have other etiologies. Such etiologies might be the stretching of the brachial plexus that can occur by the forces of labor (uterine contractions and maternal pushing) and—less likely—intrauterine cerebrovascular accidents (strokes), overstretching of the brachial plexus from fetal movement during the pregnancy, or spontaneous mal-development of the brachial plexus.
In some brachial plexus injuries sympathetic nerve fibers that traverse T1 can be damaged. This can result in depression of the eyelid and drooping of the mouth on the affected side, a constellation of symptoms called Horner's Syndrome.
click on
image to view larger image
The natural history of brachial plexus injury
Fortunately, most brachial plexus birth injuries are transient. The majority of such injuries resolve by three months, with a range of 2 weeks to 12 months. Only 4 to 15% result in some degree of permanent injury as reported by various authors in the list below:
Johnson (1979) 7.8%
Sandmire (1988) 11.8%
Nocon (1995) 4%
Eckert (1997) 5-22%
Graham (1997) 20%
Chauhan (2014) 8%
Average: ~10%
Even though shoulder dystocia occurs in only 0.5% to 1.5% of all deliveries, the fact that there are approximately 3 million vaginal deliveries a year in United States means that many hundreds of babies will experience permanent brachial plexus injury. A little math tells the story:
—If the rate of occurrence of shoulder dystocia is approximately 0.5 to 1.5%, and
—If the rate of brachial plexus injury is 10% in these deliveries, and
—If the rate of permanent injury is 10% of all brachial plexus injuries, then the rate of permanent brachial plexus injury will be one in 6,666-20,000 vaginal deliveries
This means that there will be approximately 150-450 babies born each year in the United States with permanent brachial plexus injuries. In addition, there will be babies who will suffer severe central neurologic injury such as cerebral palsy from asphyxia. There will even be babies who will die following severe shoulder dystocias. It is for these reasons that shoulder dystocia injuries have become an important area of medical — and medical-legal — concern.
Treatment options and prognosis
As mentioned, the majority of brachial plexus injuries will resolve spontaneously over the course of several months to a year. Physical therapy is usually employed within weeks of birth to help strengthen muscles whose nerve supply has been damaged. For those injuries that are permanent there are two modes of therapy.
First, physical therapy can strengthen muscles that are only partially denervated, strengthen surrounding muscles to compensate for functional loss and improving the range of motion of the affected shoulder, arm, elbow, or hand.
Second, surgical therapy in the form of nerve grafting or muscle transposition may be undertaken. There is, however, great controversy about the efficacy of such surgical procedures in improving the outcome of those with brachial plexus injuries. Several orthopedic and neurosurgeons from around the country who do this sort of surgery frequently report various degrees of improvement in many of their patients. Others in the field, however, refute these claims and feel that there is little or no benefit to such surgery.
�
Other neonatal injuries following shoulder dystocia deliveries
Fractured clavicle
The second most common injury suffered by infants following shoulder dystocia deliveries is a fractured clavicle. The incidence of this injury following shoulder dystocia is 10%.
If the fetal shoulders and chest are relatively large in relation to the maternal pelvis, significant pressure may be placed on them as they pass through the birth canal following delivery of the fetal head. In some infants, this pressure causes the clavicle to fracture. The overlapping of the ends of the broken clavicle reduces both the length between the shoulders and the diameter of the fetal chest and may allow the shoulders and chest to deliver. This "safety valve" effect may in fact help reduce the incidence of severe brachial plexus injuries.
The baseline clavicular fracture rate for all deliveries appears to be about 0.3%. Despite the fact that shoulder dystocia increases the risk of clavicular fracture 30 fold, approximately 75% of clavicular fractures are not associated with shoulder dystocia. Interestingly, although there are multiple reports of brachial plexus injuries following cesarean sections, clavicular fractures following cesarean sections are extremely rare.
Fractured humerus
This occurs in approximately 4% of infants with shoulder dystocia deliveries. While they may occur spontaneously, they are often the result of maneuvers employed to resolve a shoulder dystocia such as delivery of the posterior arm (see below). Such injuries heal rapidly and by themselves rarely result in litigation.
Contusions
The force with which an infant's shoulder is compressed against the maternal pubic bone and the pressure of the deliverer's hands on a fetus while performing various maneuvers to effect delivery will often result in bruises on the baby's body. Such bruising has often been cited by plaintiff attorneys as evidence that a baby has been handled roughly at delivery. In fact, such bruises are common even in routine deliveries not involving shoulder dystocia or fetal injury.
Fetal asphyxia
The most feared complication of shoulder dystocia is fetal asphyxia. It has been frequently demonstrated in both animal experiments and in retrospective analyses of babies born following dramatic cessation of umbilical blood flow (placental abruption, uterine rupture) that if babies are not delivered within five to 10 minutes they will suffer irreversible neurologic damage or death. Wood, in an often-quoted article from 1973, showed that in the time between delivery of the head and trunk of an infant, a baby’s umbilical artery pH declines at a rate of 0.04 units per minute. This would mean that at the five-minute mark following delivery of the fetal head, a baby's pH may have dropped from 7.2 — a common level after several hours of pushing — to 7.0, the level that defines asphyxia. By 10 minutes the pH would have dropped to 6.8.
Ouzounian (1998) reported that of 39 babies whose deliveries involved shoulder dystocia, 15 who suffered brain injury averaged a head-to-shoulder delivery interval of 10.6 minutes while the 24 babies also born following shoulder dystocia but without brain injury had a head-to-shoulder delivery interval of only 4.3 minutes.
Leung, in a more recent study—2011—found the drop in pH to be 0.011 per minute of head-to-body delivery interval as opposed to Wood’s 0.04/min. Both Leung (2011) and Lerner (2011) have shown that the risk of asphyxia during management of a shoulder dystocia delivery becomes significant at the 4-5 minute mark.
Cerebral palsy and fetal death are rare but unfortunately not unheard of consequences of prolonged head-to-shoulder delivery intervals following shoulder dystocia deliveries.
The reason for the increasing acidosis and asphyxia that occurs during a shoulder dystocia delivery is that once the fetal head emerges from the mother, the baby's umbilical cord becomes tightly compressed between its body and that of the mother's birth canal. This significantly decreases or totally cuts off blood flow between the mother and infant. If the pressure on the cord is not rapidly relieved, the consequences of lack of umbilical flow — decreased delivery of oxygen to the fetus — may occur.
Menticoglou (2016) has recently proposed a new explanation as to why resuscitation may fail in some neonates after shoulder dystocia deliveries: hypovolemic shock. He notes that not only oxygen is interrupted by compression of the umbilical cord during shoulder dystocias, but fluid and blood flow cease as well. The fetal heart can pump blood out through the thicker arterial walls of the umbilicus but the thinner venous walls may collapse, not allowing oxygenated blood to return to the fetus from the mother. If substantiated, this phenomenon may lead to a change in how babies are resuscitated after severe shoulder dystocias.
Some authors—for instance Westgate (2011)—feel that the differences in cord arterial pH seen among infants following shoulder dystocia deliveries more likely reflect the condition of a fetus in labor prior to the occurrence of a shoulder dystocia rather than the deterioration over time during the shoulder dystocia resolution process.
� Maternal injuries
The mother, too, is at some risk when a shoulder dystocia occurs. The most common complications she may suffer are excessive blood loss and vaginal and vulvar lacerations.
Significant maternal blood loss, which occurs in one quarter of all shoulder dystocia deliveries, may be seen either during the delivery or in the postpartum period. Its usual causes are uterine atony or lacerations of the maternal birth canal and surrounding structures. Such lacerations may involve the vaginal wall, cervix, extensions of episiotomies, or tears into the rectum. Uterine rupture has also been reported.
Because of the pressure directed upwards towards the bladder by the anterior shoulder when a shoulder dystocia occurs, post-partum bladder atony occurs frequently. Fortunately, it is almost always transient. Occasionally the mother's symphyseal joint becomes separated or the lateral femoral cutaneous nerve damaged, most likely the result of overaggressive hyperflexion of the maternal legs during attempts at resolving the shoulder dystocia.
Can shoulder
dystocia be anticipated accurately?
The answer to this question by the vast majority of experts in obstetrics is “No”. This is confirmed by:
The ACOG Bulletin 40 (2002, reaffirmed 2015) which says “Shoulder dystocia is most often an unpredictable and unpreventable obstetric emergency.
The ACOG publication Neonatal Brachial Plexus Palsy (2014), p. 17: “Risk factors for shoulder dystocia are not reliable predictors for its occurrence.”
Williams Obstetrics (25th Edition, 2014): “Identification of individual instances [of shoulder dystocia] before the fact has proven to be impossible.…. Most cases of shoulder dystocia cannot be accurately predicted or prevented.”
In the past, there have been physicians who have claimed that shoulder dystocia could be predicted. Hassan (1988) stated,
"In the majority of cases shoulder dystocia can be anticipated. Risk factors include maternal obesity, diabetes, preeclampsia, prolonged gestation, and fetal macrosomia. A male infant is at a greater risk for macrosomia and dystocia."
O'Leary, in his 1992 book, Shoulder Dystocia and Birth Injuries, concurred.
However, this has been an overwhelmingly minority opinion. The vast majority of obstetricians, including those who have done the most work on shoulder dystocia and brachial plexus injuries, have concluded that it is impossible with any degree of certainty to predict in which deliveries shoulder dystocia will occur. The key issue involved is "certainty". As will be shown, there are multiple "risk" factors for shoulder dystocia. Mothers and babies having these risk factors are, in an absolute sense, more likely than mothers and babies without these factors to experience shoulder dystocia. But whether the predictive value of such factors as currently measured is high enough to be useful clinically, that is, to justify changes in labor management in hopes of avoiding shoulder dystocia, is what is at issue.
Moreover, as with most statistical questions in medicine, the predictability of shoulder dystocia has to be looked at from two points of view:
Sensitivity: Are the risk factors associated with shoulder dystocia able to accurately identify most babies who will experience a shoulder dystocia at birth?
Positive predictive value: What percentage of mothers and babies having these risk factors will, in fact, experience shoulder dystocia?
In the case of shoulder dystocia, its infrequent rate of occurrence (0.5%-1.5%) and the low positive predictive value risk factors for it have severely impeded the ability of obstetricians to utilize such information to advantageously alter clinical care.
The medical literature confirms this overwhelmingly.
Resnick (1988), discussing the ability of obstetricians to predict when shoulder dystocias will occur, stated that "the diagnosis [of shoulder dystocia] will often be made only after delivery of the fetal head."
Geary (1995) reported that when all antenatal risk factors for shoulder dystocia were taken into account, the positive predictive value was less than 2% for individual factors and less than 3% when multiple factors were combined.
Lewis (1998) noted that only 25% of shoulder dystocia cases had at least one significant risk factor.
Gherman (2002), among current leaders in the study of shoulder dystocia, has said the following:
“Most of these preconception and prenatal risk factors have extremely poor positive predictive values and therefore do not allow the obstetrician to accurately and reliably predict the occurrence of shoulder dystocia.”
The obstetrical literature contains many other articles which share this point of view.
The general consensus in obstetrics is that both the sensitivity and positive predictive value for predicting shoulder dystocia is far too low to justify obstetrical interventions in hopes of avoiding it.
However, the above dictum has been challenged, particularly for shoulder dystocia with brachial plexus injury. There has been work by Emily Hamilton et al in Montreal using statistical methods to estimate the risk of shoulder dystocia with brachial plexus injury. The assessment of risk is based on the size of both the baby and the mother. This work indicates that it is possible to identify a small subgroup with very elevated risk of shoulder dystocia with brachial plexus injury, where the tradeoff between potential prevention and unnecessary intervention matches or exceeds the results using the ACOG intervention criteria.
The original algorithm evaluated the following factors: previous vagina birth, mother’s height and weight, gestational age, and estimated fetal weight. In a 2006 paper, Dyachenko and Hamilton showed that their algorithm was able to detect 50.7% of the cases of shoulder dystocia with some brachial plexus injury along with a false positive rate of only 2.7%. In a second study published in 2012 (Daly, 2012), the clinicians employed a similar algorithm prospectively, in just under 9000 deliveries from two New Jersey hospitals. Use of the algorithm resulted in a lowering of the rate of shoulder dystocia by 56.8% while not at all increasing the rate of primary cesarean sections.
Whether or not the Hamilton algorithm will change the current consensus in obstetrics that shoulder dystocia is unpredictable awaits further verification.
� Categories of risk factors
The risk factors for shoulder dystocia can generally be divided into three categories:
Preconceptual — before pregnancy
Antepartum — during pregnancy
Intrapartum — during labor and delivery
A. Preconceptual risk factors for shoulder dystocia
1. Previous shoulder dystocia
Having had a shoulder dystocia in a previous delivery proves to be the most accurate predictor for recurrence of a shoulder dystocia. This makes perfect sense. The pelvic anatomy of a woman does not change in between pregnancies. Moreover, second and subsequent babies are likely to be larger than first or previous babies.
The risk of a woman having a repeat shoulder dystocia once having had one, as reported by various authors, is:
Smith (1994) 12%
Ginsburg (2001) 11%.
Gherman (2002) 11.9%
Mehta (2007) 10%
This compares with the baseline risk for shoulder dystocia of 0.5%-1.5%. Because of this significant increase in risk -- approximately 10-fold -- some obstetricians have proposed "once a shoulder dystocia, always a cesarean".
2. Maternal obesity
A mother's weight, likewise, proves to be significantly correlated with shoulder dystocia.
Emerson (1962) showed that shoulder dystocias occurred twice as often in obese women as in normal weight women: 1.78% versus 0.81%.
Sandmire (1988) estimated that the relative risk of shoulder dystocia in women with a prepregnancy weight of greater than 82 kg (181 lbs) was 2.3.
Similar findings have been published by Hope (1998), Robinson (2003), and Kim (2014).
These reports, of course, beg the question as to whether or not obesity itself is risk factor for shoulder dystocia or whether it just reflects the fact that obese women are more likely to have macrosomic babies. Robinson (2003) studied this issue and found that maternal obesity was not significant as an independent risk factor for shoulder dystocia after adjusting for confounding variables. He found, as have others, that fetal macrosomia was the single most powerful predictor. Mehta (2014) also addressed this issue. He performed a multivariate logistic regression on the role of maternal obesity in shoulder dystocia and found, as had Robinson, that after considering other variables, obesity was not an independent risk factor for shoulder dystocia
Moreover, the literature has not shown the utility of using maternal weight to try to predict those women who will experience a shoulder dystocia at delivery. For instance, Hernandez (1990) showed that even in women weighing over 250 lbs., the rate of shoulder dystocia was no more than 5%. Thus any intervention that would have been undertaken based solely on maternal weight would have been without justification in 19 of 20 patients in his series.
There is a caveat, however. Given that more pregnant women than ever are obese, and that obesity has a marked correlation with fetal macrosomia — a known major risk factor for shoulder dystocia — it is likely that the continuing rise in the rate of maternal obesity will result in an increase in the occurrence of shoulder dystocia over the next decade.
3. Maternal age
Some studies have claimed that maternal age is a risk factor for shoulder dystocia. In one report from 2015, Zuarez-Easton reported that maternal age greater than 35 years has a 2.7 odds ratio for obstetrical brachial plexus injury.
But careful analysis reveals that maternal age is a risk factor for shoulder dystocia only in so far as maternal obesity, diabetes, excessive maternal weight gain, and instrumental deliveries are all more common in older women. These, of course, are all themselves risk factors for shoulder dystocia. In one of the few studies looking at the correlation between maternal age and shoulder dystocia in isolation, Bahar (1996) did not find any difference in shoulder dystocia based on maternal age alone.
4. Abnormal pelvis
O'Leary, in his book on shoulder dystocia, places great significance on the abnormal pelvis as a risk factor for shoulder dystocia -- but offers no data to support his claim. Although it would make sense that a decrease in certain pelvic dimensions would increase the possibility of a baby's anterior shoulder getting caught on the maternal pubic bone, there are no reports in the literature demonstrating a relationship between shoulder dystocia and objectively-measured pelvic shape.
Moreover, the use of pelvimetry in obstetrics-- x-ray or other measurements of pelvic dimensions – was, for the most part, discarded years ago, for several reasons:
1. Except in the most extreme cases of congenital or pathological pelvic deformity, there is poor correlation between pelvic size and a woman's capacity to delivery vaginally.
2. The ability to more accurately monitor babies in labor enables obstetricians to safely allow labor itself to be the test of whether or not a baby will "fit" into and through the maternal pelvis.
5. Multiparity
In a 10-year series collected from Boston's Beth Israel Hospital covering the years 1975 to 1985, Acker (1988) showed that there were more Erb palsies in babies born to multiparous women then to primigravida women. He attributed this to a marked increase in precipitous labors in such women. In his series he noted that 31.8% of all babies with Erb palsy had experienced a precipitous delivery. Overland (2012) confirmed these findings. Acker felt that this correlation between precipitous deliveries and shoulder dystocia was due to the fetus’s shoulders—in precipitous deliveries—not having time to align themselves in the oblique as opposed to the A-P orientation, thus predisposing them to shoulder dystocia.
Additionally, as with maternal age, by the time a woman becomes "multiparous", she is old enough to have an increased risk of having other risk factors for shoulder dystocia such as larger babies, obesity, and diabetes. Moreover, only multiparous women could have the very significant risk factor of having had a previous shoulder dystocia. Thus most experts feel any relationship between multiparity and shoulder dystocia is secondary to other, more primary, risk factors.
6. Gestational age
Paradoxically, Overland in a 2013 study showed that, after adjustment for birth weight, there is a consistent reduction in the risk of shoulder dystocia with increasing length of pregnancy. That is, per pound of baby the risk of shoulder dystocia was higher at 36 weeks than 40 weeks and higher at 40 weeks than 41 weeks. This trend was particularly pronounced in pregnancies complicated by maternal diabetes.
Summary of preconceptual risk factors
- Previous shoulder dystocia significantly increases the risk of repeat shoulder dystocia
- Shoulder dystocia is seen more commonly with increased maternal age, obesity, and multiparity -- but in reality these are only markers for the increase of other primary risk factors
- There is no evidence linking the "abnormal pelvis" to shoulder dystocia
B. Antepartum factors risk factors for shoulder dystocia
1. Macrosomia
Other than a history of a previous shoulder dystocia, macrosomia is far and away the most significant risk factor for this condition. It is the factor that has been most studied and most often proposed as a potential target for manipulation in hopes of reducing the number of shoulder dystocia deliveries. Some authors go so far as to claim that no other risk factor has any independent predictive value for the occurrence of shoulder dystocia.
The most obvious confirmation of this relationship consists of those studies measuring the percentage of babies in different weight groups that experienced shoulder dystocia. What is vitally important to keep in mind when considering such data, however, is that these are the weights ascertained after delivery. They were not available to the obstetrician before delivery in making his or her clinical decisions as to how the delivery should be conducted.
Acker (1985) found that babies weighing over 4500gms experienced shoulder dystocia 22.6% of the time. The shoulder dystocia rate in his general population was 2%. His report showed the following:
|
Infant
weight in Nondiabetic women |
Percent shoulder dystocia |
|
Less than 4000 g
|
1.1% |
|
4000g - 4499 g |
10.0% |
|
Greater than 4500 g |
22.6% |
More than 70% of all shoulder dystocias in his study occurred in infants weighing more than 4000 g.
Lazer (1986) reported that the shoulder dystocia rate for infants weighing more than 4500 g was 18.5% while for "smaller babies" in his series the rate was 0.2%.
Nisbet (1998) published a chart showing similar data:
|
Infant Weight
|
Percent shoulder dystocia |
|
4000-4250 |
5.2 |
|
4250-4500 |
9.1 |
|
4500-4750 |
14.3 |
|
4750-5000 |
21.1 |
Sandmire (1998) likewise showed that the incidence of shoulder dystocia significantly increased with birth weight:
|
Infant weight |
Rate of shoulder dystocia |
| Less than
4000g |
0.3% |
| 4000-4500 g
|
4.7% |
| Greater than
4500 g |
9.4% |
Vidarsdottir (2011) studied 41,000 deliveries in Iceland where babies generally tend to be large. Of the 41,000 neonates in his study, 343 were “extremely macrosomic (>5000 gms). This represented 0.9% of all deliveries. The odds ratio for shoulder dystocia in this group was 26.9. There were 46 shoulder dystocias among the 343 extremely large babies (14%).
Tsur (2012) evaluated 240,000 deliveries in Israel and determined that the odds ratio for shoulder dystocia in patients with macrosomia (defined as 4 kg) compared to babies weighing less than 4000gm was 16.1.
Revicky (2012) in England evaluated 9767 vaginally deliveries at 37 weeks or more between 2005 and 2007. The incidence of shoulder dystocia was 2.4%. The only independent risk factors for shoulder dystocia in his review were birthweight and instrumental delivery.
Cheng (2013) reviewed the medical records of 80,953 singleton deliveries at Prince of Wales Hospital in Hong Kong between 1995 and 2009. The incidence of macrosomia was 3.4%. The overall incidence of shoulder dystocia was 0.3%. The incidence rose with increasing birth weight. The odds ratio for shoulder dystocia with a birth weight of 4000 to 4199 g was 22.4 while the odds ratio for birth weight of 4200 g or more was 76.1.
Overland (2014) looked at this issue in a huge series of 1,914,544 deliveries. He found that 75% of all cases of shoulder dystocia occurred in deliveries of offspring weighing 4000 g or more. The association was slightly stronger in parous women than in primigravidas.
Parantainen (2014) evaluated 42,964 deliveries in Finland and reported that a baby with a birth weight of over 4000 g has a relative risk of 12.1 for shoulder dystocia compared to a population of lesser sized babies.
Temerinac (2014) found that in the weight interval 2500 – 4000, the rate of shoulder dystocia was 1.4% but that in babies bigger than 4500gm the rate was 16.2%.
Mehta (2014) showed that the incidence of shoulder dystocia increases with each 500 g of birth weight, reaching a tenfold increase by 4500 g.
Callaghan (2014) found adjusted odds ratios for shoulder dystocia of 15, 52, and 157 for birth weights of 4 – 4.5 kg, 4.5 – 5 kg, and greater than 5 kg respectively.
Hehir (2015) published a paper in which he showed that 17 of 120 infants with a birth weight of greater than 5000 g had a shoulder dystocia for a rate of 14.2%. Three of these suffered an Erb palsy, all of which resolved.
Macrosomia also seems to increase the rate of injuries following shoulder dystocia:
Jackson (1988) showed in his series of 8258 deliveries that the average birth weight of babies who suffered brachial plexus injuries was 4029 g. whereas the average birth weight of all noninjured deliveries was 3160 g,
Kolderup (1997), in a review of the delivery of 2924 macrosomic babies at UCSF, reported that macrosomic infants had a six fold increase in significant injury from shoulder dystocia deliveries compared with controls.
What is macrosomia?
The definition of macrosomia has varied both through the years and according to the author(s) writing about it. The various cutoff points used to define macrosomia have been 4000 g, 4250, 4500 g, and 5000 g. Often a distinction has been made between macrosomia in nondiabetic versus diabetic mothers, the bar being set lower for the fetuses of diabetic mothers.
ACOG, in the new 2016 Bulletin on Macrosomia (#173), defines macrosomia this way:
At this time, it seems reasonable to recognize a continuum of risk and to divide macrosomia into three categories:
Birth weight of 4,000–4,499 g with increased risk of labor abnormalities and newborn complications
Birth weight of 4,500–4,999 g with additional risk of maternal and newborn morbidity
Birth weight of 5,000 g or greater with additional risk of stillbirth and neonatal mortality
The 25th edition of the Williams Obstetrics textbook (2014), on the other hand, says:
We are of the view that the upper limit of fetal growth, above which growth can be deemed abnormal, is likely two standard deviations above the mean, representing perhaps 3% of births. At 40 weeks, such a threshold would correspond to approximately 4500 g.
One of the most important factors about macrosomia is the differential rate of growth of the fetal head, chest, and trunk as gestation proceeds, both in the babies of diabetic and of nondiabetic mothers. Until 36-38 weeks, the fetal head generally remains larger than the trunk. Between 36 and 40 weeks, however, the relative growth of the abdomen, chest, and shoulders begins to exceed that of the head. This is especially the case in babies of diabetic mothers where glucose substrate levels are higher in both the mother and fetus. Thus both in prolonged gestation and in babies of diabetic mothers the size of a baby's shoulders and trunk is likely to increase relative to the head, increasing its chances of shoulder dystocia.
How is fetal weight predicted and how accurate are these predictions?
Although the correlation between fetal weight and shoulder dystocia is of great interest to obstetricians, knowing about this relationship is of no use unless fetal weight -- and the corresponding increased risk of shoulder dystocia -- can be predicted prior to delivery. How good, therefore, are our current techniques for estimating fetal weight?
Traditionally, fetal weight has been estimated by measurement of uterine height and by Leopold maneuvers. "Leopold maneuvers" is the name given to palpation of the maternal abdominal wall with a series of four specific steps in order to determine fetal position, fetal presentation, and to estimate of the size of the baby.
Such estimates, however, are notoriously inaccurate. Studies have shown grave discrepancies between estimation of fetal weight by experienced obstetricians and actual infant weight at delivery. Moreover, multiparous women are often as accurate in their estimates of fetal weight as are clinicians and ultrasonic examinations (Chauhan, 1992).
With the advent of ultrasonic fetal evaluation in the 1970's, it was hoped that a more accurate means of assessing fetal weight was at hand. Many papers were published presenting formulas for ultrasound estimation of fetal size based on measurement of various fetal parameters. Most of these involved some combination of measurements of the fetal head, abdominal dimensions and fetal femur length. However comprehensive analyses of these various ultrasound formulas have concluded that none are consistently more accurate than being within 10 to 15% of actual birth weights. Chauhan in 1995 went so far as to say that in more than half of the models for ultrasound prediction, clinical predictions by obstetricians were as or more accurate. This was found to be especially true for larger babies:
From these data it appears that sonographic models are not significantly superior to clinical examination in detecting newborns with birth weights greater than or equal to 4000 g.
There are many studies that confirm the inability of any current diagnostic technique to determine fetal weight prior to delivery to a range any better than 10-15% above or below the true birth weight.
Benson (1987): The use of ultrasound formulas to predict macrosomia was correct in only 47% of infants; the positive predictive value was only 36-43 per cent.
Delpapa (1991): Only 48% of estimates of fetal weight as determined by ultrasound within three days of birth were within 500 g of the final fetal weight.
Jazayeri (1999): Using a formula based on ultrasound-measured abdominal circumference in an attempt to determine which babies would weigh over 4500gm, the positive predictive value was only 9%.
Rossi in 2013 summarized the literature between 2000 and 2012 on the topic of prenatal identification, management, and outcomes of macrosomic infants. He found that
1. Both clinical and sonographic examinations are poorly predictive of macrosomia.
2. Knowledge before delivery that a neonate might weigh more than 4000 g does not improve neonatal outcomes.
3. Ultrasound has poor sensitivity in the detection of macrosomia: Between 9.4% and 15.3% in detecting birth weights greater than 4000 g. and between 6.3% and 30.4% for detecting a birth weight greater than 4500 g.
Burkhardt (2014), in a study of 12,794 deliveries, found that the mean percentage error of weight estimation by ultrasound was 8.8% in babies that had shoulder dystocia and 4% in a control group.
Shoulder/chest/abdomen ratios
As discussed above, both post-term growth and maternal diabetes result in the fetal trunk growing larger than the fetal head. The same pattern of disproportionate growth occurs with babies that are large for any reason, including inherent genetic predisposition. This is why macrosomic babies have a higher incidence of shoulder dystocia. In a normally proportioned baby, once the head is delivered the fetal shoulders and body usually emerge from the vagina easily. With shoulders and trunk bigger than the fetal head, however, it is more likely that they will get stuck.
Several investigators have sought to measure the differences in size between fetal shoulders, trunk, and head circumference to see if there existed a certain ratio at which the risk of shoulder dystocia became prohibitively high.
Hopewood (1982) proposed that when the transthoracic diameter is 1.5 cm larger than the biparietal diameter, shoulder dystocia would be significantly increased.
Kitzmiller in 1987 developed a formula involving a CT scan of fetal shoulders by which he was able to predict fetal weight with improved accuracy: a positive predictive value of 78% for predicting birth weights over 4200 g. with a negative predictive value of 100%.
Cohen (1996) found that an abdominal diameter minus biparietal diameter measurement of greater than or equal to 26 mm was highly discriminative in the detection of shoulder dystocia and correlated well with incidence and severity.
However, several authors have refuted the utility of using the relationship between measurements of different anatomic structures to predict shoulder dystocia.
Benson (1986), while acknowledging that femur length:abdominal circumference ratios differ in macrosomic vs. nonmacrosomic fetuses, claimed that there is too much overlap between the larger and smaller groups in any formula protocol to be clinically useful. He states in his paper that "for no cutoff value of these measurements is there a high sensitivity and high specificity."
Melendez (2009) showed that fetal abdominal circumference measurements of greater than 35 cm can be used to identify more than 90% of macrosomic infants—but also demonstrated that this method had a low positive predictive value in detecting specific cases of shoulder dystocia.
Burkhardt (2014), in an evaluation of almost 13,000 deliveries, found that there was a significant difference in
--abdominal diameter
--abdominal circumference
--abdominal diameter minus biparietal diameter
--abdominal circumference minus head circumference
between shoulder dystocia and control deliveries. Unfortunately, the positive predictive value when applying the proposed cut off for abdominal diameter minus biparietal diameter of 26 mm was only 7.6%. Burkhardt thus concluded that these measurements are not applicable as screening tools for predicting shoulder dystocia.
Thus the question: Can shoulder dystocia be reliably predicted by estimating fetal weight?
The problems with attempting to estimate which fetuses will be macrosomic and using this information as a tool for predicting shoulder dystocia are twofold:
In the first place, it is the general conclusion of most obstetrical experts who have studied this issue that predicting macrosomia is unreliable. If macrosomia cannot be reliably determined, it is hard to try to use it to predict shoulder dystocia.
Secondly, only a very small percentage of babies, even of those who have macrosomia, go on to develop shoulder dystocia. This presents a significant obstacle to the use of estimates of fetal weight as a tool for deciding when to change clinical management in hopes of preventing shoulder dystocia deliveries.
These difficulties are highlighted in the data presented below:
Resnick (1980) found that shoulder dystocia occurred in only 1.7% of 1409 infants born at Johns Hopkins Hospital weighing more than 4000 g.
Acker (1986) pointed out that although the relative frequency of shoulder dystocia varied directly with increasing birth weight, almost half of the shoulder dystocias occurred in deliveries involving average and smaller babies. This is because there were so many more of them. Forty-seven percent of all shoulder dystocias at the Beth Israel hospital during the time of his study occurred in babies weighing less than 4000 g, a weight category which encompassed 91.2% of the total delivery population. Thus any attempt to use estimates of fetal weight as an isolated factor to reduce the incidence of shoulder dystocia would miss half of all shoulder dystocias -- even if macrosomia could be accurately measured.
Delpapa's 1991 study showed that, at his institution, more than half of babies estimated to weigh more than 4000gm in fact had birth weights below 4000gm -- a false positive rate for predicting macrosomia of >50%.
Levine in 1992 showed that if macrosomia was defined as the 90th percentile of fetal weight for a given gestational age, then sonographic prediction of macrosomia was wrong 50% of the time both in underestimating and overestimating fetal weight.
Geary (1995) found that the positive predictive value of a birth weight of more than 4000 g for predicting shoulder dystocia was only 3.3%.
Gonen (2000) evaluated 17 babies with brachial plexus injuries from a population of 16,416 deliveries. Only three of these injured babies were macrosomic.
Hansen (2014), in a review of literature on this topic, found that 27% of babies who experienced shoulder dystocias weighed less than 4000 g.
Burkhardt (2014) studied 12,794 vaginal deliveries and found that the majority of shoulder dystocia --56%--occurred in non-macrosomic fetuses
The American College of Obstetricians and Gynecologists bulletin on shoulder dystocia states that ultrasound has a sensitivity of only 22 to 44% and a positive predictive value of only 30 to 44% in predicting macrosomia.
Similar unsuccessful attempts to accurately ascertain fetal birth weight during the antenatal or intrapartum period have been published by Boyd (1983), Levine (1992), Chauhan (1992), Sandmire (1993), and Sacks DA (2000)
As the above data confirms, the general consensus of obstetricians who have done research in the area of shoulder dystocia is that the occurrence of shoulder dystocia based on estimations of fetal weight cannot be reliably predicted.
El Madany sums up this issue well in his 1990 paper:
Even if certain combinations of risk factors exist which could with high likelihood isolate which babies experienced shoulder dystocia, the inability to predict macrosomia with the requisite degree of certainty on which such a clinical suspicion is based precludes making active action protocols. Until the macrosomic infant can be accurately identified, no reasonable risk factor profile can be established.
Sandmire, in his 1993 article, concludes:
Any approach using ultrasound would have to demonstrate that its use improves newborn or maternal outcome without disproportionate increases in morbidity and mortality. A barrier to achieving this goal is the inaccuracy associated with ultrasonic estimations of fetal weight. The current ultrasonic procedures for estimation of fetal weight are not accurate enough for detecting macrosomia defined by weight criteria. And even if clinicians could determine fetal weight accurately, the frequency of persistent fetal injuries associated with vaginal birth of the macrosomic fetus is so low that induction of labor or cesarean birth is not justified on that basis. Delivery decisions based on inaccurate estimated fetal weight should be avoided.”
Thus, while macrosomia is a major risk factor for shoulder dystocia, it has not been possible to accurately predict shoulder dystocia by attempting to predict which babies will be macrosomic.
click on
image to view larger image

2. Diabetes
Next to macrosomia, the factor most closely associated with shoulder dystocia is maternal diabetes in pregnancy. The prevalence of diabetes in pregnant women is increasing due to an older pregnant population, a higher rate of obesity, and more thorough antenatal detection (Young 2013).
One of the first clear-cut demonstrations of this was Acker's 1985 paper showing the following:
|
Estimated fetal wt. |
Nondiabetic mothers
% shoulder dystocia |
Diabetic mothers
% shoulder dystocia |
| < 4000 g
|
1.1% |
3.7% |
| 4000-4499 g |
10.0% |
30.6% |
| > 4500 g |
22.6% |
50% |
As can be seen, babies of diabetic mothers had a three to fourfold increase in the risk of shoulder dystocia compared to babies of nondiabetic mothers in each weight category.
Although diabetic mothers accounted for only 1.4% of the birth population in this study, they accounted for 4.9% of shoulder dystocias. Acker also showed that although the general rate of Erb palsy following shoulder dystocia is roughly 10%, 17% of babies born to diabetic mothers developed Erb palsy.
Other investigators have shown similar or larger correlations between diabetes and shoulder dystocia:
Sandmire (1988) found a relative risk for shoulder dystocia in the babies of diabetic mothers of 6.5 compared to nondiabetic mothers.
In Al-Najashi's 1989 study, the rate of shoulder dystocia in babies weighing over 4000gm born of diabetic mothers was 15.7%. Babies born to nondiabetic mothers had a shoulder dystocia rate of 1.6%.
Casey (1997), in a study of over 62,000 patients, found the shoulder dystocia rate in his general obstetrical population to be 0.9% while in his patients with gestational diabetes it was 3%.
Tsur (2011), in a study from Israel, showed that the odds ratio for shoulder dystocia with diabetes was 1.8 compared to nondiabetic mothers.
Overland (2013), in a population of just under 2 million deliveries in Norway, reported that shoulder dystocia occurred in 0.73% percent of all deliveries but 3.95% of deliveries in which the mother had diabetes.
Mehta (2014): Diabetes increases the overall risk of shoulder dystocia by more than 70%. In his study, the incidence of macrosomia was 21% among diabetic mothers versus 7.6% among those who were nondiabetic. Gram for gram, the incidence of shoulder dystocia and injury is higher in diabetic mothers.
Hansen (2014) reported that in his patient population the ratio of shoulder dystocia of nondiabetic to diabetic mothers was 0.6%:1.9%, a 201% increase.
There are two main reasons for this correlation between diabetes and shoulder dystocia. In the first place, diabetes in pregnancy is strongly linked to macrosomia. The growth of babies of diabetic mothers represents not only their genetic potential for growth but also reflects the conversion to fat of the excess glucose substrates present in both mother and baby. Secondly, as previously mentioned, growth is not as evenly distributed between the head and trunk in the babies of diabetic mothers as it is in those of nondiabetic mothers. Rather, babies of diabetic mothers show a pattern of greater shoulder, chest, and abdominal growth. As Ellis summarized in 1982:
The infant of a diabetic mother has a different body configuration than the infant of a nondiabetic mother. Increased deposition of fat in various organs may be due to increased insulin secretion in response to hyperglycemia.
Can shoulder dystocia be predicted in babies of diabetic mothers?
In the 1980s several authors published studies purporting to show that they could predict which babies of diabetic mothers would be at high risk for shoulder dystocia.
Elliott (1982) claimed that by evaluating the chest and biparietal diameters in infants of diabetic mothers weighing more than 4000 g, he could reduce the incidence of traumatic morbidity at delivery from 27% to 9%.
Tamura (1986) found that in diabetic women fetal abdominal circumference values greater than the 90th percentile correctly predicted macrosomia in 78% of cases. In his study, when both the abdominal circumference and the estimated fetal weight exceeded the 90th percentile in pregnant women with diabetes, macrosomia was correctly diagnosed 88.8% of the time.
Mintz, in a promising study from 1989, published data showing that in his hands a combination of fetal abdominal circumference greater than the 90th percentile for gestational age and shoulder soft tissue width greater than 12 mm was the best predictor of macrosomia. His data reported a sensitivity of 96%, specificity of 89%, and "accuracy" — positive predictive value — of 93%. He also found a significant correlation between shoulder width and a high HgA1C (a blood test that measures blood sugar control over the preceding three months).
Unfortunately, these results have not been supported or replicated by other investigators. Multiple experts in the field of shoulder dystocia have published data from very large series that contradict the conclusions listed above. In addition, the results of the above studies are not as powerful as might first be assumed.
In Elliott's study, for instance, although he was able to show that a large number of babies meeting certain chest-biparietal diameter criteria were macrosomic, 39% of babies with these same parameters — chest/biparietal diameter ratio of > 1.4 — were not larger than 4000 g. In Tamura's study, although he was able to predict macrosomia in babies meeting certain abdominal circumference criteria, he still was unable to identify the vast bulk of macrosomic fetuses. As for Mintz's study, no one has yet been able to duplicate his results.
In fact, most studies have found that neither macrosomia nor shoulder dystocia can be reliably predicted in the babies of diabetic mothers.
Acker (1985) showed that by using the criteria of large baby and diabetic mother he could predict 54.7% of shoulder dystocias — but would miss 45.3% of them (false negatives).
Delpapa (1991) stated that the predictive value of estimated fetal weight in babies of diabetic mothers for predicting shoulder dystocia was not sufficiently accurate to reliably identify them.
Moreover, most diabetic mothers do not have macrosomic babies and the overwhelming majority of macrosomic infants are not babies of diabetic mothers.
There are two other studies of interest relating to this question.
Coen (1980) showed that although HgbA1C is a good marker for long-term monitoring of blood sugars in diabetic patients, it is not a good predictor of large-for-gestational age infants. The average HgA1C in mothers of large-for-gestational age infants in his study was 6.7; for mothers delivering normal sized babies the average HgA1C was 6.5 — too close to be clinically useful.
Casey (1997) reported that although the rate of shoulder dystocia was in fact increased in mothers with gestational diabetes, this was not manifest in an increase in the rate of Erb palsy.
The bottom line is that macrosomia is as difficult to predict in diabetic mothers as it is in the nondiabetic population.
� 3. Maternal weight gain
The data linking maternal weight gain and fetal birth weight are controversial.
Abrams (1995) and Langhoff-Roos (1987) both showed that total maternal weight gain was significantly correlated with infant birth weight.
Dawes (1991), however, was not able to confirm this:
There was no apparent correlation between maternal weight gain and birth weight between women giving birth to average for gestation or large for gestational age infants
Several other investigators have reported conflicting information as to the effect of patterns of maternal weight gain on ultimate fetal weight. Some studies have found second trimester weight gain to be the major determinate whereas others have found that the weight gain in the last trimester was the most important factor. Given the contradictory and confusing data on this subject, Dawes' closing statement is probably the most apt:
The variations in total (maternal) weight gain and incremental weight gain are so wide that these measurements are unlikely to be clinically useful.
4. Fetal sex
There is little data correlating fetal sex with macrosomia and shoulder dystocia. Although on average male babies do weigh slightly more than females, there is no data showing a significantly higher number of macrosomic male infants than female infants.
Resnick in his classic 1980 paper mentions fetal sex as a potential factor but does not supply data to substantiate his claim.
El Madany (1990) showed that 59.2% of babies experiencing shoulder dystocia in his study were male — statistically significant but not of much value as a clinical predictor.
� 5. Post-dates
Even though fetal growth slows in the last several weeks of pregnancy, there is still some growth as long as pregnancy continues. Thus the longer the baby remains in utero, the larger the baby will be — and the greater the risk of shoulder dystocia. Acker (1985) was one of the first to demonstrate this association. Chervenak confirmed this in 1989 when he reported that 25.5% of babies delivering at 41 weeks gestation were macrosomic while only 6% prior to 41 weeks were (risk ratio 4.2) in a group delivering between 38 and 40 weeks gestation. Hernandez (1990), too, found a direct correlation between post-date babies and an increased risk of shoulder dystocia. He attributed this entirely to the increased tendency of post-date babies to be macrosomic.
Overton in 2013 looked at this question in greater detail. He found that without correcting for weight, the rate of shoulder dystocia at 36 weeks is 27% of that at 40 weeks. However, after correcting for birth weight, the relative risk of shoulder dystocia at 36 weeks — compared to 40 weeks — was 1.68. Thus after adjustment for birth weight his results showed that there was a consistent reduction in the risk of shoulder dystocia from 36 weeks onward. This finding was particularly pronounced in pregnancies complicated by maternal diabetes.
Summary of antepartum risk factors
- Macrosomia and maternal diabetes are the main risk factors for shoulder dystocia
- Predicting fetal weight is extremely unreliable
- Other factors — maternal weight gain, fetal sex, and post dates — are secondary risk factors. They are correlated with an increased risk for shoulder dystocia but are only relevant to the degree that they increase the risk of fetal macrosomia
- Since multiparity increases the number of precipitous labors it may be a slight primary risk factor for shoulder dystocia
� C. Intrapartum risk factors
Various characteristics of labor and delivery have been claimed to be useful in predicting whether or not a given mother-baby pair will end up with a shoulder dystocia and possible brachial plexus injury.
1. Instrumental delivery
Several studies have clearly shown that labors that end in instrumental vaginal deliveries — vacuum or forceps — show a higher rate of shoulder dystocia in each fetal weight group.
Nesbitit (1998), for example, reported the following data:
|
Weight (g) |
SD % in unassisted births |
SD % in instrumental
deliveries |
| 4000-4250 |
8.4% |
12.2% |
| 4250-4500 |
12.3% |
16.7% |
| 4500-4750 |
19.9% |
27.3% |
| >4750 |
23.5% |
34.8% |
Baskett (1995) similarly showed a tenfold increase of shoulder dystocia and a fivefold increase in brachial plexus injury (BPI) with mid-forceps deliveries
| |
SD |
BPI |
|
SVD |
0.3% |
0.04% |
|
Low forceps deliveries |
0.9% |
0.06% |
|
Midforceps delivery |
2.8% |
0.5% |
Benedetti (1978) reported that in deliveries with the combination of a prolonged second stage of labor and a mid-pelvic delivery there was a 4.6% rate of shoulder dystocia -- compared to 0.4% when there was neither a prolonged second stage nor a mid pelvic delivery.
McFarland (1986) showed that the relative risk of brachial plexus injury was 18.3 for midforceps deliveries and 17.2 for vacuum deliveries when compared to unassisted vaginal deliveries.
Hansen in 2014 reviewed the literature on shoulder dystocia with assisted vaginal deliveries. He found a shoulder dystocia rate of 0.6% with spontaneous deliveries but a rate of 2.0% with operative vaginally deliveries, a relative difference of 254%.
Parantainen (2014), in a Finnish study of 42,964 deliveries with 152 shoulder dystocias, found a relative risk of 3.98 between spontaneous deliveries and vacuum assisted deliveries.
Mehta (2014) found that shoulder dystocias increased by 35 to 45% in vacuum and forceps-assisted deliveries. For nondiabetic mothers with assisted deliveries this translated to shoulder dystocia rates of 8.6% for infants weighing 4000 to 4250 g, 12.9% for infants weighing 4250 to 4500 g, 23% 4500 to 4750 g, and 29% for infants 4750 to 5000 g. The total adjusted odds ratio for shoulder dystocia with instrumentally assisted deliveries was 1.9.
Zuarez-Easton (2015) reached a similar conclusion; he found an OR of 3.6 between spontaneous and vacuum assisted deliveries in which there was a brachial plexus injury.
Is there a difference between the use of forceps or vacuum when it comes to increasing the risk of shoulder dystocia?
Bofill (1997) found that there was a non-significantly higher incidence of shoulder dystocia with vacuum assist versus forceps: 4.6% versus 1.9%.
Dall’Asta (2016), on the other hand, showed no difference in the rate of shoulder dystocia between the use of vacuum and forceps. He postulated that the use of the vacuum or forceps to expedite fetal head delivery may interfere with the spontaneous mechanism of rotation of the trunk and ultimately with the descent of the shoulders in the birth canal. The lack of difference in his study between forceps and vacuum, as compared to Bofill’s study, may perhaps be attributed to the implementation of safer vacuum equipment since 1997.
Thus it is clear that deliveries requiring instrumental assistance have a higher risk of shoulder dystocia and brachial plexus injury than do spontaneous vaginal deliveries. It is not clear, however, that it is the instrumental deliveries themselves that are to blame for these shoulder dystocias. It may well be that the mother's inability to push the baby out without assistance is due to fetal macrosomia, an altered distribution of fat between the fetal head, chest, shoulders, and abdomen, or descent of the shoulders in the A-P as opposed to an oblique orientation-- themselves major risk factors for shoulder dystocia.
2. Experience of the deliverer
Since the safe resolution of a shoulder dystocia involves specific obstetrical maneuvers and since shoulder dystocias occur relatively infrequently, it would seem that more experienced practitioners would have better outcomes in these situations merely by virtue of having seen more of them. Such an opinion would surely be voiced by most obstetricians and experienced labor and delivery nurses. However the data does not support this belief.
Acker in 1988 looked at the experience of the deliverer in relation to neonatal injuries following shoulder dystocia deliveries. He found that the number of Erb palsies following shoulder dystocias did not vary with either the number of years a physician had been in practice or the number of deliveries that physician performed. As Acker stated,
Most clinicians hardly gain expertise and confidence in the difficult manipulations required to resolve shoulder dystocia due to the rarity of the condition.
3. Labor abnormalities
Several studies have shown a higher incidence of shoulder dystocia in labors in which the second stage of labor is prolonged. Nevertheless -- and paradoxically -- shoulder dystocias are not infrequently seen during labors with very rapid second stages.
Hopewood (1982) found that there was a deceleration phase of active labor between eight to 10 cm in 58% of shoulder dystocia deliveries.
Acker (1985) showed that arrest disorders significantly increase the chance of shoulder dystocia with larger babies.
Gross (1987) showed that a prolonged deceleration phase and long second stage contributed to brachial plexus injury risk but that these were only weak predictors.
Al-Natasha (1989) found that both a delay in the second stage of labor and slowed descent of the fetal head in obese multiparous women greatly increased the possibility that a shoulder dystocia would occur.
Weizsaecker (2007) found that brachial plexus injury is often but not always preceded by dysfunctional labor. In his study, active phase abnormalities predominated among the mothers who experienced a shoulder dystocia. The most important risk factor for shoulder dystocia was a long deceleration phase. This increased the adjusted odds of brachial plexus injury almost 6 fold.
Tsur (2011) showed that the odds ratio for shoulder dystocia with slow rate of descent during the second stage of labor was 2.4.
But the literature has sometimes contradicted itself on this issue.
Acker, in that same 1985 article referenced above, states:
No particular labor abnormality was predictive of an increased incidence of shoulder dystocia relative to that encountered with a normal labor pattern, a spontaneous delivery, or both.
McFarland (1975) likewise reported the same rate of labor abnormalities of the active phase of labor and of the second stage of labor in both shoulder dystocia and control groups. He concluded that labor abnormalities could not serve as clinical predictors for the subsequent development of shoulder dystocia.
Hernandez (1990) reported that although there is a relationship between the length of various stages of labor and shoulder dystocia, 70% of patients who experienced shoulder dystocia had normal labor patterns.
Lurie (1995) found no correlation between the length of the stages of labor and shoulder dystocia. He showed that there was no difference in (1) the mean rate of dilatation, (2) the percentage of protracted labors, or (3) the mean duration of the second stage of labor in a group of mothers who experienced shoulder dystocia deliveries versus a group that delivered without complication. His conclusion was that protracted labor did not seem to be a risk factor for shoulder dystocia. As he says in his paper,
One could not identify shoulder dystocia in advance while analyzing the rate of cervical dilation or duration of the second stage of labor.
Even if disorders of labor were found to be correlated with shoulder dystocia, it is not clear whether this would represent an independent risk factor. It might merely confirm that labor disorders are more common with macrosomic babies and that macrosomic babies more commonly experience shoulder dystocia. To date there has been no major study evaluating the length of various stages of labor broken down by neonatal weight categories in relationship to shoulder dystocia deliveries.
To further complicate the issue, it is well known—as discussed above--that shoulder dystocias and brachial plexus injuries are often seen with short second stages of labor:
Acker (1988) found that 31.8% of all babies with Erb palsy were born after precipitate second stages of labor. As he explains,
The rapidity of descent may prohibit the fetal shoulders from entering the inlet in an oblique diameter, preclude adequate preparation for delivery, and add to nerve root trauma.
Gonen (2000) reported that 7 of 17 patients (41%) with brachial plexus injury had second stages of labor shorter than 10 minutes
� 4. Oxytocin and anesthesia
There does not appear to be any independent correlation between the use of either oxytocin or anesthesia and shoulder dystocia deliveries.
Oxytocin is generally used to increase the strength of uterine contractions. To the extent that oxytocin has to be used more frequently with macrosomic infants, it might have a secondary correlation with shoulder dystocia deliveries. But there is no published data linking oxytocin use with the incidence of shoulder dystocia independent of fetal weight.
Likewise with anesthesia; there is no reported increase in shoulder dystocia deliveries in labors in which conduction anesthesia is employed.
5. Episiotomy
There is no statistically significant relationship between the absence of episiotomy, the frequency of shoulder dystocia, and any subsequent neonatal injury. That this is the case is perplexing given that almost all protocols for the resolution of shoulder dystocia advocate making a "generous episiotomy". This recommendation appears to be without support in the obstetrical literature.
Gurewitsch (2004) demonstrated that management of severe shoulder dystocia with an episiotomy versus fetal manipulation alone or both does not influence neonatal depression rates.
Paris (2011) reviewed a total of 94,842 births in which there were 953 shoulder dystocias and 102 brachial plexus injuries. The rate of episiotomy with shoulder dystocia dropped from 40% in 1999 to 4% in 2009 with no change in the rate of brachial plexus injury per 1000 vaginally births.
Sagi-Dain in a 2015 review of 14 articles on the subject found no evidence supporting the use of episiotomy in the prevention and management of shoulder dystocia.
There are two possible reasons one might make an episiotomy in the case of a shoulder dystocia.
The first would be to make more room for the baby to emerge. In this situation the indications for making an episiotomy would be the same as in any delivery: alleviating soft tissue dystocia of the perineum. If the perineal tissue were tight, then an episiotomy might be helpful in delivering the baby. However, if the soft tissue of the vagina and vulva is pliable and stretches easily, as in most multiparous women, then an episiotomy will not make it any easier to free the anterior shoulder from behind the pubic bone.
The second possible indication for an episiotomy during a shoulder dystocia would be to allow more room for the obstetrician's hand to reach inside the vagina in order to perform rotational maneuvers or to attempt to deliver the baby's posterior arm. An episiotomy might be helpful in accomplishing these maneuvers in a woman whose perineal tissues impede access to the fetal shoulders. However, in a woman in whom the perineal tissues are lax enough to allow these maneuvers to be performed, the automatic making of an episiotomy will not facilitate delivery and would be unnecessary.
Thus the almost universal recommendation that an episiotomy be made during all shoulder dystocia deliveries is without literature or data support.
Combination of risk factors
As would be expected, several studies have shown that a combination of risk factors significantly increases the risk of shoulder dystocia.
Benedetti in 1978 published an article noting that the combination of macrosomia greater than 4000 g, prolonged second stage of labor, and mid pelvic operative vaginally delivery led to a 21% incidence of shoulder dystocia and a high rate of neonatal injury.
Mehta (2014) noted that in the setting of fetal macrosomia and a second stage of labor greater than two hours, performance of assisted vaginally delivery led to an increase rate of shoulder dystocia.
Busoni found that the combination of birth weight greater than 4000 g and vacuum delivery led to an odds ratio of 13.7 for shoulder dystocia; for birth weight over 4500 g with use of the vacuum the odds ratio was 21.5.
The greatest risk for shoulder dystocia occurs in those groups of women who have multiple risk factors. An obese woman with a large pregnancy weight gain and gestational diabetes will have a significantly greater likelihood of having a macrosomic baby and shoulder dystocia than will a woman who has just one of these risk factors. The worst possible combination of risk factors would be an obese mother with diabetes, an estimated large-for-gestational-age fetus, a prolonged second stage of labor, and a forceps delivery. The rate of shoulder dystocia in such a situation would approach 40%.
So, Can shoulder dystocia and brachial plexus injury be predicted?
There are some authors who have always felt that shoulder dystocia can be prevented. O'Leary, in his book on shoulder dystocia, states:
A well-prepared obstetrician or midwife can anticipate this problem [shoulder dystocia] as a result of routinely identifying those risk factors that predispose to shoulder dystocia. Thus prevention requires identification of risk factors, which leads to anticipation of the problem . . . Identification of critical risk factors will lead to anticipation, which in turn will lead to prevention.
O'Leary then boldly goes on to say:
The presence of macrosomia of 4500 g alone is justification for cesarean section in nonobese women. The presence of macrosomia of 4000-4500 g may in itself be sufficient to warrant abdominal delivery when other risk factors, especially a platypoid (flat) pelvis, diabetes and/or obesity, are present.
But despite the certitude of his statements, O'Leary presents no data to support his recommendations.
Other authors have also tried to articulate guidelines for avoiding shoulder dystocia. Anchor (1988) has said:
We advocate the abdominal mode delivery for infants of diabetic gravidas whose best estimated fetal weight exceeds 4000 g.
Langer (1991) stated that if all infants of diabetic mothers who weighed 4250 g or more were delivered by cesarean section, the overall cesarean section rate would increase by only 0.26% while shoulder dystocia would be reduced by 76%. He goes on to acknowledge, however, that in the nondiabetic group there is no weight that provides an optimal threshold for cesarean section to avoid shoulder dystocia.
But statements such as these have represented the fringe of obstetrical opinion. It has been the consensus of the vast majority of obstetricians who have studied the subject that there is no real way to figure out which babies are likely enough to have shoulder dystocia to warrant changes in the management of their labors.
The basic issue is this: One can suspect shoulder dystocia all one wants. But is there some combination of factors that predicts shoulder dystocia with an accuracy great enough to make doing cesarean sections, performing early inductions, or making other changes in management a reasonable course of action? The answer by most experts in the field of shoulder dystocia has been "No." Certainly there are risk factors which do increase the odds of shoulder dystocia and brachial plexus injury occurring. But so many babies with each of these risk factors do not encounter shoulder dystocia and brachial plexus injury that it is difficult to justify changes in management of all labors on the basis of these suspicions.
The majority of studies in the obstetrical literature have not been able to show that the sensitivity or positive predictive value of various methods for predicting shoulder dystocia is high enough to justify interventions--which usually means cesarean section. While macrosomia, diabetes, prolonged second stage of labor, instrumental delivery, and other factors do indicate a statistically increased risk of having a shoulder dystocia, their low positive predictive value and high false positive rate make them clinically useless as tools for predicting -- and hence trying to prevent -- shoulder dystocia.
The entire issue is best summed up in Practice Bulletin #40 "Shoulder Dystocia" (2002, reaffirmed 2015) by the American College of Obstetricians and Gynecologists. They find the preponderance of current evidence consistent with the following positions:
Most cases of shoulder dystocia cannot be predicted or prevented because there are no accurate methods to identify which fetuses will develop this complication.
Ultrasonic measurement to estimate macrosomia has limited accuracy
Planned cesarean section based on suspected macrosomia is not a reasonable strategy
Planned cesarean section may be reasonable for the nondiabetic with an estimated fetal weight exceeding 5000 g or the diabetic whose fetus is estimated over 4500 g
Supporting the position of the American College of Obstetricians and Gynecologists on the lack predictability of shoulder dystocia are the thoughts of various shoulder dystocia investigators
Resnick (1980): Most babies with shoulder dystocia do not have risk factors. "The diagnosis will often be made only after delivery of the fetal head."
Acker (1986): Almost half (47.6%) of all shoulder dysoticas occurred in babies weighing less than 4000 g.
Al-Najashi (1989) 41% of shoulder dystocia deliveries in his series occurred in infants of average birth weight, that is 2500 to 3999 g.
Basket (1995): The profile of risk for shoulder dystocia -- prolonged pregnancy, prolonged second stage of labor, macrosomia, and assisted mid-pelvic delivery -- was not clinically useful because "the large majority of cases of shoulder dystocia occur in patients without these risk factors"
Rouse and Owen (1996) used a theoretical model involving performing cesarean section in women with suspected macrosomic fetuses in order to prevent permanent brachial plexus injury. They defined macrosomia as 4500 g. Their model predicted that 3695 cesarean sections would be needed to prevent one case of permanent injury.
Eckert (1997): The greatest number of injuries occurred in nondiabetic patients with birth weights of less than 4000 g.
Lewis (1998): Only 25% of shoulder dystocia cases had at least 1 significant risk factor . . . the positive predictive value of pre-partum risk factors for shoulder dystocia is less than 2% individually, 3% when combined.
Irion, in a 1998 Cochrane Systematic Review, indicated that there was no benefit in terms of improved maternal or infant outcomes associated with the induction of labor for suspected fetal macrosomia. He also noted that cesarean sections are not without risk. He calculated that if in a given country an additional 10,000 cesarean sections were performed in an attempt to prevent shoulder dystocia, there would likely be 900 severe postpartum hemorrhages, the need for 100 blood transfusions, and 600 each of wound infections, endometritis, and urinary tract infections. There also would likely be an additional 30 cesarean hysterectomies 35 women with venous thromboembolism, 30 women with severe morbidity requiring admission to intensive care, one hundred women with uterine rupture in a subsequent pregnancy were vagina birth to be attempted, and at least one additional maternal death.
Gonen (2000) showed that 740 cesarean deliveries were needed to prevent a single case of permanent neurologic damage if all mothers suspected to have fetuses weighing greater than 4500 g underwent elective cesarean section.
Gherman (2002): "Most of these preconception and prenatal risk factors have extremely poor positive predictive values and therefore do not allow the obstetrician to accurately and reliably predict the occurrence of shoulder dystocia."
Chauhan (2004): Due to inaccuracies in predicting fetal weights, among uncomplicated pregnancies suspicion of macrosomia is not an indication for induction or for primary cesarean section.
Cunningham, author of Williams Obstetrics (22nd edition, 2005) reports that 99.5% of babies weighing 4000-4500 gms had a safe vaginal delivery without shoulder dystocia.
Backe (2008) evaluated 30,574 births, 91 of which were diagnosed with brachial plexus injury, 15 of which were permanent. Although he identified various risk factors--shoulder dystocia, macrosomia, diabetes, vacuum extraction, and forceps delivery—their predictive power was poor. He concludes that “plexus injury is not well predicted by known risk factors”.
Nath (2012): 233 out of 241 patients treated at Texas nerve and paralysis Institute for brachial plexus palsy had shoulder dystocia at delivery. 80% of the patients in the study were not macrosomic. Instrumental use was 41%. Higher birth weight does not affect the prognosis of brachial plexus injury.
Dodd in 2012 showed that while there are a number of factors associated with an increased risk of shoulder dystocia, none are of sufficient sensitivity or positive predictive value to allow their use clinically to reliably and accurately identify the occurrence of shoulder dystocia. While they did find that maternal diabetes, induction of labor, and infant birthweight greater than 4000 g was associated with an increased risk of shoulder dystocia, they are poorly predictive of shoulder dystocia at a population level. The findings of the study reinforce the occurrence of shoulder dystocia as in “unpredictable and unpreventable obstetric emergency”. He notes that the Royal College of Obstetricians and Gynecologists does not recommend cesarean delivery for the prevention of shoulder dystocia.
Parantainen (2014) estimated that at least 30 unnecessary cesarean sections would be required to prevent one shoulder dystocia when using an optimal cut off for the most accurate ultrasound parameters for estimating fetal weight: abdominal diameter minus biparietal diameter of greater than 25 mm. Moreover, in his series the birth weight for shoulder dystocia babies was less than 4000 g in 35% of cases.
Peleg’s (2015) institution had a policy of counseling women about risks when there was a sonographically estimated fetal weight of greater than 4000 g. Their study was unable to show that a policy of elective cesarean for macrosomia significantly reduced the incidence of either shoulder dystocia or brachial plexus injury.
Palatnik (2016) attempted to predict the occurrence of shoulder dystocia prior to assisted vaginal deliveries by identifying significant risk factors and combining them into a prediction model. These factors included multiparity, maternal diabetes, chorioamnionitis, arrest of labor or maternal exhaustion, use of vacuum, and an estimated fetal weight of greater than 4000 g. Unfortunately, this model did not allow the accurate prediction of shoulder dystocia. The area under the receiver operating characteristic curve was 0.73, demonstrating only a modest ability to predict shoulder dystocia before performing an operative vaginally delivery.
Sentilhes (2016) discussed the guidelines for clinical practice of the French College of Gynecologist and Obstetricians. These guidelines state that according to the literature, only two characteristics are independent risk factors for shoulder dystocia: a history of a previous shoulder dystocia (which multiplies the risk by 10 – 20) and fetal macrosomia (risk multiplied by 6 – 20). Diabetes and maternal obesity are also consistently associated in the literature with an increased risk of shoulder dystocia (on the order of 2-4 times higher). But these associations are explained, at least in part, by the macrosomia they induce. The existence of a direct effect of maternal diabetes or obesity on this risk, independently of fetal weight, remains to be demonstrated. Nonetheless, even the factors associated continually and independently with shoulder dystocia do not enable its reliable prediction because they are not sufficiently discriminant. From 50 to 75% of all cases of shoulder dystocia occur in their absence, and the vast majority of deliveries in which they are present not involve it. Shoulder dystocia therefore remains an unpredictable obstetrical emergency.
As mentioned above, however, these conclusions may soon have to be changed. As the studies by the Hamilton group have shown, evaluation of multiple factors related to shoulder dystocia by means of a carefully researched algorithm seem to be able to predict with an impressive degree of certainty those fetuses at risk for shoulder dystocia and brachial plexus injury at the cost of no increase in the cesarean section rate.
Are there any strategies that can reduce the chances of a shoulder dystocia occurring?
Since shoulder dystocia is known to be associated with macrosomic fetuses and risk is increased in babies of diabetic mothers, various strategies have been proposed utilizing this knowledge to attempt to decrease the incidence of shoulder dystocia and hence related brachial plexus palsies. Let us examine some of these proposed strategies
Would elective cesarean section for suspected macrosomia be a reasonable strategy for decreasing the number of shoulder dystocias and brachial plexus injuries?
Many papers have been written trying to assess the utility of performing cesarean sections for suspected macrosomia in an attempt to reduce the risk of shoulder dystocia and permanent brachial plexus injury.
Gonen (2000) studied the use of physical examination and ultrasound during labor to identify babies suspected of being greater than 4500 g. His goal was to see if by performing cesarean sections in these cases he could reduce the rate of permanent brachial plexus injury. Macrosomia was suspected in 47 cases -- but was only confirmed at cesarean delivery in 21 of these (45% positive predictive value). Thus there were 26 unnecessary cesarean sections due to a false diagnosis of macrosomia. Moreover, over 84% of the macrosomic babies born from his subject population were missed. Of the 115 cases of true macrosomia in his series, only 21 were correctly identified in labor -- a dismal sensitivity rate of 18.3%. Of the 17 babies that developed brachial plexus injuries in his study, three were macrosomic -- but they were not identified prior to or during labor! The remaining 14 injured babies were not macrosomic.
Thus, Gonen's attempt to decrease the brachial plexus injury rate by performing cesarean sections on suspected macrosomic babies missed most big babies and resulted in many unnecessary cesareans. He confirmed what is a major problem with any attempt to predict and prevent shoulder dystocia and brachial plexus injury: The group in which they occur most often is that of normal sized babies.
Many other studies have resulted in similar conclusions:
McFarland (1986) presented data by weight group showing how many cesarean sections would need to be performed to prevent even temporary brachial plexus injury:
|
Estimated wt |
# C/S's |
| >4500 g |
165 |
| 4000-4500 g
|
1383 |
His conclusion is that even if a reliable means of estimating fetal weight were possible, by performing cesarean sections for all babies estimated to be greater than 4500 g only 32% of shoulder dystocias would be avoided. At any lower weight cut off, there would be far too many cesarean sections for far too little gain.
Delpapa (1991) studied nondiabetic women thought via ultrasound to have macrosomic fetuses. He concluded that he would have to do 76 cesarean sections to prevent five cases of shoulder dystocia. If the rate of permanent brachial plexus injury is 1 in 100 shoulder dystocias, that would mean 7600 cesarean sections to prevent 1 permanent injury.
Sandmire’s 1993 article discussed in some detail the difficulty of attempting to determine fetal size in utero. Any approach using ultrasound would have to demonstrate that its use improves newborn or maternal outcome without disproportionate increases in morbidity and mortality to mother and baby. A barrier to achieving this goal is the inaccuracy associated with estimation of fetal weight. According to Sandmire, ultrasonic procedures for estimation of fetal weight are not accurate enough for detecting macrosomia.
He goes on to say that even if clinicians could determine fetal weight accurately, the frequency of persistent fetal injuries associated with vaginal birth of the macrosomic fetus is so low that induction of labor or cesarean birth is not justified on that basis. Delivery decisions based on what are likely to be inaccurate estimated fetal weights should be avoided.
Sandmire also drew up a chart drawn from data in several other studies in which he evaluated the rate of permanent brachial plexus injuries and the number of cesarean sections that would be necessary to avoid them:
|
Study |
C/S to prevent BPI injuries |
C/S to prevent permanent
BPI injury |
|
Gordon (1973) |
526 |
10,520 |
|
Sandmire (1988) |
(no data) |
7403 |
|
McFarland (1986) |
1922 |
39, 840 |
|
Modanlou (1980) |
588 |
11,700 |
Sandmire reiterated his thinking in another paper published in 1996. In it he concludes that a policy of employing cesarean section for suspected macrosomia in hopes of preventing permanent brachial plexus injury will not work because of:
1. The inaccuracy of ultrasound in estimating fetal weight
2. The increases in morbidity and mortality that would occur from the very large numbers of cesarean sections so generated.
3. The many cesarean sections that would have to be done to prevent one significant fetal injury
Sandmire also takes care to distinguish minor injuries, such as clavicular fracture and transient brachial plexus injury, from severe persistent fetal injuries. He recommends that anyone considering the issue of cost vs. benefit in the management of suspected macrosomia should make decisions based only on significant fetal injuries, such as permanent brachial plexus injuries and severe neurologic damage.
Several other authors have concurred with Sandmire’s conclusions:
Basket (1995) stated that if in his series of patients all mid-forceps deliveries had been replaced by cesarean sections, 3268 cesarean section deliveries would have been performed to prevent 16 non-permanent brachial plexus injuries. Even if cesarean sections were performed only for babies suspected of being greater than 4500 g, 54 cesarean sections would have to be performed to prevent one case of non-permanent brachial plexus injury.
Eckert, in his 1997 paper, confirms the problems described by previous authors: In practice, only estimates of fetal weight, not actual weights, are available to clinicians seeking to predict the risk of birth injury. Weights estimated before delivery, whether by ultrasound or clinical estimation, are notoriously inaccurate. Even if we were able to identify a specific fetal weight that mandated cesarean section, any scheme that relied on estimated fetal weight to “risk” patients into cesarean delivery would result in the delivery of many infants appreciably smaller than the estimated fetal weight assigned them.
Eckert points out that the greatest number of injuries occurred in nondiabetic pregnancies with birth weights less than 4000 g.—and no protocol for managing macrosomia recommends cesarean delivery for an estimated fetal weight of less than 4000 g. Eckert concludes:
In our opinion, the number of cesarean sections necessary to prevent a single birth injury in a normal glycemic population precludes our recommending mandatory cesarean delivery at any weight cutoff. Our study does not support the contention that elective cesarean section is justified in those women with fetuses suspected to be macrosomic as a means of preventing persistent infant mortality. A very large number of unnecessary cesarean sections would be performed without much preventive effect.
Kolderup (1997) found that a policy of elective cesarean section for macrosomia would necessitate 148 to 258 cesarean sections to prevent a single persistent injury. He feels that "these findings support a trial of labor and judicious operative vaginal delivery for macrosomia infants."
Bryant’s data (1998) showed that even assuming ultrasound diagnosis to be accurate in predicting fetal weight, between 155 and 588 cesarean sections would have to be performed to obviate a single case of permanent injury, depending on the weight cut-off selected:
Our data show that a policy of elective cesarean delivery in cases of suspected fetal macrosomia had an insignificant effect on the incidence of brachial plexus injury. Although the contribution of this policy to the cesarean delivery rate was small, the number of cesarean deliveries required to prevent a single case of permanent brachial injury was high and probably unjustified.
Gregory (1998) stated that if 5.5% of all brachial plexus injuries were permanent -- which his data demonstrated -- only one in 3833 macrosomic infants would have a persistent Erb palsy. Moreover, he found that one half of all of the shoulder dystocias in his series occurred in normal weight infants.
Rouse and Owen (1999) quantified the effectiveness of a policy of elective cesarean section for ultrasound-diagnosed fetal macrosomia. They found that in women without diabetes, if a cesarean section were performed for each baby with a suspected weight of greater than 4500 g, 3695 cesarean sections would have to be performed for each permanent brachial plexus injury prevented.
Homer (2011) evaluated 591 extremely obese women in England between 2007 and 2008 He found no significant differences in anesthetic, postnatal, or neonatal complications between women with planned vaginally delivery and planned cesarean delivery with the exception of shoulder dystocia--3% versus 0%. None of the infants with shoulder dystocia suffered permanent brachial plexus injury. The study does not provide evidence to support a routine policy of cesarean delivery even for extremely obese women. The entrance criteria for the study was a BMI of equal to or greater than 50.
Summarizing, the major conclusion of most of the obstetrical literature discussing the strategy of performing cesarean sections for suspected macrosomia is that it would not be practical because it would require far too many unnecessary interventions for the benefits that would be obtained. As noted, the new work by Hamilton’s group may over the next several years invalidate these conclusions.
Very importantly, there is one more issue that needs to be addressed in discussing the above question. It is that
Cesarean sections are not without risk, especially for diabetic and/or obese women
Although cesarean section is one of the most commonly performed operations in the United States, it still carries much greater risk for the mother than does a vaginal delivery. These risks include blood loss, infection, damage to other pelvic organs, and respiratory emergencies. Moreover, the recovery period following a cesarean section is longer and more painful than after a vaginal delivery, and performing one cesarean section greatly increases the likelihood that a woman will have her next baby by cesarean section as well. Finally, total hospital care for women delivering a baby via cesarean section is 50%-100% more expensive than the cost of a vaginal delivery.
Thus in order to justify the increased risk, pain, and expense of performing a cesarean section in hopes of avoiding shoulder dystocia and permanent brachial plexus injury, there has to be substantial evidence that this is an effective policy. As has been shown, such evidence is currently lacking. In fact, the evidence has been contrary to this supposition.
What about early inductions as a means of avoiding shoulder dystocia and brachial plexus injury?
Many have thought that by cutting off one to two weeks of growth of a fetus at term, a baby might be born at a lighter weight than if delivered at term. This difference in newborn weight might be enough to avoid shoulder dystocia and the risk of permanent brachial plexus injury. Is this a viable policy?
In the first place, the growth rate of babies differs significantly, both between babies and at various points in pregnancy for each baby. Thus it is impossible to estimate how much additional growth is prevented by "early delivery". Moreover studies testing this hypothesis—until very recently—have been disappointing.
Del Papa (1991) found that early induction did not decrease infant morbidity.
Gonen (1997) randomized patients suspected of macrosomia based on ultrasound examination to an early induction group -- 134 patients -- or a routine pregnancy follow-up group -- 139 patients. There was no statistically significant difference in shoulder dystocia between the two groups.
Several authors -- Leaphart (1997), Friesen (1995), Combs (1993) -- have even shown that this approach of early induction actually increased the cesarean section rate with no decrease in the incidence of shoulder dystocia.
Sanchez-Ramos (2002) reviewed 11 studies with 3751 subjects, 2700 of whom were managed expectantly while 1051 underwent labor induction. Compared with those whose labor was induced, women who experienced spontaneous onset of labor had a lower incidence of cesarean section (RR 0.39) and higher rates of spontaneous vaginally delivery (RR 2.07). No differences were noted in the rates of operative vaginally delivery, incidence of shoulder dystocia, or abnormal Apgar scores. Sanchez-Ramos’s summary: labor induction for suspected fetal macrosomia results in an increase cesarean delivery rate without improving perinatal outcomes.
Thus until recently there was no data to support a policy of early induction in an attempt to decrease the rate of shoulder dystocia. More recent literature, however, seems to show that this such a policy might, in fact, have some benefit
Boulevain in 2015 compared induction of labor with expectant management for large for dates fetuses to try to prevent shoulder dystocia. His trial ran between 2002 and 2009 in centers in France, Switzerland, and Belgium. Women suspected of carrying a macrosomic fetus were divided into 2 groups, one in which women were induced between 37 and 38 6/7 weeks of gestation, the other receiving expectant management.
Boulevain’s findings contradicted those of all previous studies: In his series, induction of labor substantially reduced the risk of shoulder dystocia and associated morbidity compared with expectant management (RR 0.32). The rate of significant shoulder dystocia in the induction of labor group was 1% while in the expectant management group it was 4%. The risk ratio for “any” shoulder dystocia—as opposed to “significant” shoulder dystocia--was 4% compared with 8% for a risk ratio of 0.47. Moreover, the cesarean section rate in the induction group was 28% versus 32% for the expectant management group.
Can shoulder dystocia be resolved without fetal injury when it does occur? The management of shoulder dystocia
The preponderance of evidence from the literature on shoulder dystocia shows clearly that:
(1) Shoulder dystocia cannot be predicted with any degree of accuracy and
(2) Shoulder dystocia cannot be prevented by any specific strategies or maneuvers.
The question thus arises "How should shoulder dystocia be managed when it does occur? Can it be resolved successfully without injuring the baby or the mother?"
Much has been written on this subject. Multiple maneuvers claiming to be able to resolve shoulder dystocia have been described. We will now take a look at what these maneuvers are, how they are performed, and how effective they have proven to be.
Recognition
The first step in treating shoulder dystocia is recognizing when it occurs.
There are two main signs that a shoulder dystocia is present:
(1) The baby's body does not emerge with standard traction and maternal pushing after delivery of the fetal head.
(2) The "turtle sign". This is when the fetal head suddenly retracts back against the mother's perineum after it has emerged from the vagina. The baby's cheeks bulge out, resembling a turtle pulling its head back into its shell. This retraction of the fetal head is caused by the baby's anterior shoulder being caught on the back of the maternal pubic bone, preventing delivery of the remainder of the baby.
Turtle Sign

photo by Kristina Kruzan, kristinakruzan@gmail.com
Traction: "Excessive" or "Necessary" Force?
Babies rarely fall out of the pelvis -- nor should they. Especially in an age where conduction anesthesia (epidurals, spinals) is used routinely, often a mother must push several times in order to deliver the remainder of her baby after its head is born. To facilitate the passage of the baby's anterior shoulder under mother's pubic bone, it is standard practice for the deliverer to deflect the baby's head downwards. It is of interest that plaintiff lawyers in shoulder dystocia medical-legal cases and the expert witnesses they hire often say that there should be no downward traction utilized in resolving a shoulder dystocia delivery. Yet as the 2014 ACOG treatise on neonatal brachial plexus palsy states,
Because the position of the infant within the maternal pelvis will be at some angle relative to the horizontal plane (e.g., delivery table) during the final cardinal movements of labor, axial traction is generally applied in the direction or vector below the horizontal plane (also referred to as downward axial traction as distinguished from downward lateral traction or lateral bending).
It is also often said in court rooms that traction should never be applied to the fetal head during attempts to resolve a shoulder dystocia. This is certainly not the case. Unless a baby falls out of a mother—which is certainly not what happens with a shoulder dystocia—some traction—“gentle”, “moderate”—is always applied by the delivering clinician to the infant’s head. This is, in fact, the standard of care practiced by obstetricians across the United States and is the procedure describe in multiple obstetrical textbooks (see, for instance the Williams Obstetrics textbook or the Stanford Handbook of Obstetrics. Such assisting of delivery of the head is a necessary and approved obstetrical practice.
Normal
Delivery Traction

What about the slippery term "excessive force"? This term conveys an image of an obstetrician pulling with all his or her might, propping a leg against a delivery table for support, etc.
Students of shoulder dystocia have long sought to determine exactly what degree of force constitutes "excessive force". Some investigators, such as Allen (1991) and Gonik, have even tried to determine this by using specially-constructed gloves with piezoelectric fingertip sensors to measure pressures at delivery.
It would seem on the face of it that the use of strong force to attempt to deliver an impacted shoulder should be universally condemned. But one must take into account the circumstances involved. There are times when all maneuvers have been attempted to resolve a shoulder dystocia and when the only options left are either a maximal effort to extract the baby, including greater than desired forces, hypoxic neurologic damage, or fetal death. In such cases, faced with the ultimate catastrophe of the death of a baby, the risk of brachial plexus or other fetal injury must be accepted.
What the physician must not do when a shoulder dystocia occurs is to lose composure. Most shoulder dystocias occur unexpectedly. But by restraining panic, keeping a cool head, and employing a previously thought-out—and trained for--set of maneuvers, almost all shoulder dystocias can be resolved with excellent results for both baby and mother. The term "almost all" is used advisedly as sometimes, even in the most expert hands, and even with relatively mild shoulder dystocias, fetal or maternal injury will occur.
What to do when a shoulder dystocia occurs
Several things should be done as soon as a shoulder dystocia is recognized. First, the obstetrician should announce that a shoulder dystocia is present. He or she should then request that a second obstetrician called, if possible, and should ask the nurses to make sure that extra personnel are available. The obstetrician should also stay informed of the time that has elapsed since delivery of the head. One means of doing this is to designate someone to call out the time since delivery of the head at fixed intervals -- perhaps every 30 seconds. Pediatric or neonatal assistance should also be called so as to be available to evaluate and potentially resuscitate the baby after delivery. Anesthesia staff should be summoned. If sufficient staff is available, one person should be designated as a note taker to record the timing of events.
How much time does one have to resolve a shoulder dystocia before hypoxic brain injury becomes a significant risk?
In general, the operator has up to five minutes to deliver a previously well-oxygenated term infant before an increased risk of asphyxial injury occurs.
Several studies over the years have attempted to determine the drop in fetal pH in the minutes following the onset of a shoulder dystocia. As noted previously, Wood (1973) was the first to examine this question in detail. He determined that for each minute during the interval between delivery of the fetal head and trunk in a shoulder dystocia delivery a fetus’s pH drops 0.04 units per minute. Subsequent studies have challenged this data; Leung in 2011 found the rate of pH drop per minute during a shoulder dystocia to be 0.01 unit. Yet as Gherman has shown (2006), there is not a good correlation between the head-to-body delivery interval and pH, pCO2, and pO2.
More recently, both Leung (2011) and Lerner (2011) have shown that the delivering clinician has roughly 4-5 minutes to resolve a shoulder dystocia before the risk of ischemic neuropathy becomes significant. This time frame of course depends upon the oxygenation and acidosis status of the fetus prior to the onset of the shoulder dystocia.
The Maneuvers
Once a shoulder dystocia is recognized, there are several specific obstetrical maneuvers that have been proven to be of benefit in assisting in the resolution of the dystocia.
(For an excellent in-depth review of shoulder dystocia resolution maneuvers and the traction forces involves, see Stitely and Gherman [2014].)
McRoberts maneuver and suprapubic pressure
The first two maneuvers generally attempted in order to resolve a shoulder dystocia are (1) McRoberts maneuver and (2) suprapubic pressure. In fact both of these maneuvers are so benign and so effective that they are sometimes employed prophylactically in anticipation of a potential shoulder dystocia.
click on
image to view larger image

McRoberts maneuver is named for William A. McRoberts, Jr. who popularized its use at the University of Texas at Houston. It involves sharply flexing the legs upon the maternal abdomen. By doing this, the symphysis pubis is rotated cephalad and the sacrum is straightened. In a high percentage of cases this by itself suffices to free the impacted anterior shoulder.
Suprapubic pressure is the attempt to manually dislodge the anterior shoulder from behind the symphysis pubis during a shoulder dystocia. It is performed by an attendant making a fist, placing it just above the maternal pubic bone, and pushing the fetal shoulder in one direction or the other. Since shoulder dystocias are frequently caused by an infant's shoulders entering the pelvis in a direct anterior-posterior orientation instead of the more physiologic oblique diameter, pushing the baby's anterior shoulder to one side or the other from above can often change its position to the oblique which will allow its delivery. Suprapubic pressure in conjunction with McRoberts maneuver is often all that is needed to resolve 50-60% of shoulder dystocias.
In order to show more clearly how McRoberts maneuver aids in the resolution of a shoulder dystocia, Gherman (2000) performed a study in which he took x-rays of 36 women in the dorsal lithotomy position before and after McRoberts positioning. He found that there were no significant changes in the anterior-posterior and transverse diameters of the pelvic inlet, midpelvis, and pelvic outlet. There also was no increase in the obstetric, the true, and the diagonal conjugates of the pelvis. Thus, McRoberts maneuver does not change the actual dimensions of the maternal pelvis. What it does do, however, is to rotate the symphysis pubis toward the maternal head. This significantly changes the angle of inclination between the top of the symphysis and the top of the sacral promontory. This, in conjunction with the flattening of the sacrum, is often enough to allow stuck fetal shoulders to deliver.
Suprapubic
Pressure

A study by Gonik and Allen (1989) confirmed that this is the case. They showed that implementation of McRoberts maneuver can significantly reduce required fetal extractive forces and brachial plexus stretching in shoulder dystocias. In addition to allowing the anterior shoulder to slide more freely under the bottom of the symphysis, the flattening of the sacrum relative to the lumbar spine allows the posterior fetal shoulder to more easily pass over the sacrum and through the pelvic inlet.
How successful is McRoberts maneuver? Gherman (1997) observed 250 shoulder dystocia deliveries at USC from 1991 to 1994 and reported that McRoberts maneuver alone was successful in resolving 42% of them. Fifty-four percent of all shoulder dystocias were resolved by a combination of McRoberts maneuver, suprapubic pressure and/or procto-episiotomy without further maneuvers being necessary. McFarland (1996) reported similar findings: 39.5% of shoulder dystocias resolved with McRoberts maneuver alone while 58% resolved with a combination of McRoberts maneuver and suprapubic pressure.
Although McRoberts maneuver and suprapubic pressure are generally safe, it is possible to cause maternal injury by performing them. Symphyseal separations and transient femoral neuropathies from overly aggressive hyperflexing of the maternal thighs have been reported. Whether or not an infant might be subject to injury with these maneuvers has been questioned by Gurewitsch and Allen (2005)--see below.
Wood's Screw maneuver
First described in the literature in 1943, this procedure involves the progressive rotation of the posterior shoulder in corkscrew fashion to release the opposite impacted anterior shoulder. In its classic description, pressure is applied on the posterior shoulder's anterior surface. A variation of this -- the Rubin's maneuver -- involves pushing on the posterior surface of the posterior shoulder. In addition to the corkscrew effect, pressure on the posterior shoulder has the advantage of flexing the shoulders across the chest. This decreases the distance between the shoulders, thus decreasing the dimension that must fit through the pelvis. Gurewitsch and Allen (2005) feel that these maneuvers place less stretch on a fetus’s brachial plexus than do the McRoberts maneuver or suprapubic pressure.

Delivery of the posterior arm
Another effective maneuver for resolving shoulder dystocias is the delivery of the posterior arm. In this maneuver, the obstetrician places his or her hand behind the posterior shoulder of the fetus and locates the arm. This arm is then swept across the fetal chest and delivered. With the posterior arm and shoulder now delivered, it is relatively easy to rotate the baby, dislodge the anterior shoulder, and complete delivery of the remainder of the baby.
The major risk of this procedure is that of fracturing the humerus. Gherman (1998) reported 11 (12.4%) humeral fractures in 89 shoulder dystocias resolved by delivery of the posterior arm. However, since almost all humeral fractures heal quickly and without permanent damage, this would appear to be a small price to pay for the successful delivery of an infant in a life threatening situation.
Delivery of the posterior shoulder: Menticoglou and the posterior axillary sling
Menticoglou (2006) first described putting a finger into posterior axilla of the fetus to pull the posterior shoulder downward. This enables the grasping of the posterior arm, allowing it to be delivered, followed by delivery of the trunk. In 2009 Hofmeyr reported on a variation of this procedure. Instead of just using a finger, Hofmeyr recommends placing a soft plastic catheter sling around the posterior axilla and using that for traction.
Many, however, have voiced skepticism about these maneuvers. For if one has enough access to the fetal shoulder and axilla to place a finger or a sling around them, one wonders why the delivery could not have been performed as a routine rotational maneuver. Moreover, Cluver in 2015 reported on the use of the axillary sling in 19 cases. In five the baby had already died. Delivery was successful in 18 cases. There were 3 posterior humerus fractures, four cases of transient Erb palsy, and one case of permanent Erb palsy.
There have been multiple other techniques and procedures described over the years to resolve shoulder dystocias. None of these, however, have reached the level of "mainstream". Some of these are the Zavanelli maneuver, deliberate fracture of the clavicle, symphysiotomy, the "all-fours" maneuver, and fundal pressure.

Zavanelli maneuver
Although almost certainly performed by obstetricians and midwives in the past, this maneuver was first described in the obstetrical literature by Dr. Zavanelli, an obstetrician in private practice in Pleasanton, California in 1977. Dr. Zavanelli reported that during one difficult shoulder dystocia delivery, after having attempted all other maneuvers, he finally resorted to flexing the fetal head and pushing it back up into the vagina. He was then able to perform an emergency cesarean section and deliver a live baby.
The first step in any cephalic replacement maneuver (now called the Zavanelli maneuver) is to set up for an emergency cesarean section. The fetal head is rotated to the occiput anterior position and flexed. Constant firm pressure is applied while pushing the head back into the vagina. Tocolytic agents such as terbutaline, nitroglycerin, or uterine-relaxing general anesthesia may be administered to facilitate this process. Cesarean section must be performed immediately after replacement of the head.
The Zavanelli maneuver enjoys a mixed reputation. O'Leary (1993) reported on 59 women who had undergone replacement of the fetal head following unsuccessful attempts at vaginal delivery. All but 6 of these infants were successfully replaced and delivered by Cesarean section. He therefore suggested that the Zavanelli maneuver might not need to be used as a last resort maneuver but might be considered if any undue difficulty were encountered with a shoulder dystocia.
But a closer look at the data O’Leary reports is less than reassuring. In his series, the delay of cephalic replacement following delivery of the head ranged from 5 minutes to greater than 30 minutes. He was unable to replace the fetal head in six instances and he reported replacement as "difficult" in five. Apgar scores at 5 minutes were less than 6 in 61% of these babies and were less than 3 in 27%. Four babies in his series had seizures in the nursery, two had permanent neurologic injury, five experienced a permanent Erb palsy, and two died. Three percent of the mothers experienced a ruptured uterus and 5% suffered uterine lacerations.
Although Sanberg (1999) reported a much more optimistic experience with the Zavanelli maneuver -- 77.3% “success rate” involving 84 cases, neonatal complications in his series included clavicular fractures, humoral fractures, Erb palsies, quadriplegia, brain damage, mental retardation, various degrees of cerebral palsy and even mortality.
The data from these two series is sobering. While it is incumbent upon all obstetricians to know about the Zavanelli maneuver and to be able to perform it when other options for shoulder dystocia resolution have been exhausted, its significant potential for fetal and maternal injury must relegate it to the status of a "last ditch" procedure.
Transabdominal shoulder rotation (“Abdominal rescue”)
O’Shaughnessy in 1998 described another “last ditch” approach. He reported two cases in which, after unsuccessfully performing multiple shoulder dystocia resolution maneuvers, he performed a laparotomy and hysterotomy and then manually rotated the fetus’s stuck shoulders until he was able to complete a vaginal delivery.
Deliberate fracture of the clavicle
Almost all detailed accounts of shoulder dystocia include “deliberate fracture of the clavicle” as one modality for resolving this situation--but there are few accounts of this procedure actually being performed. In practice, the clavicle poses a formidable obstacle to its fracture. It is a significant bone, even in a fetus. Although the fracture of the clavicle certainly would decrease the transverse diameter of the chest and shoulders, the potential of damaging the great vessels, fetal lungs, and other structures make this an extremely hazardous procedure even if it were possible to perform easily. In fact most descriptions of transection of the clavicle involve fetuses that are already dead and require the use of a large scissors or other sharp instrument for cutting the bone.
Symphysiotomy
Symphysiotomy is a procedure that had been performed in desperate situations in the past and is now performed only in areas remote from the ability to perform Cesarean sections on a rapid basis. The theory is that by transecting the firm ligaments joining the left and right symphyseal bones, an additional 2-3cm in pelvic circumference can be gained. In most cases this will allow the anterior shoulder of a stuck fetus to be delivered beneath the symphysis. The benefit of the procedure is that it can be performed rapidly -- it usually takes 5 minutes or less -- and can be done under local analgesia. In subsequent pregnancies a woman who has undergone a symphysiotomy has an intact uterus and a slightly enlarged pelvis.
The symphyseal separation obtained by symphysiotomy affects the transverse diameters of the pelvis, particularly those of the mid cavity and outlet. The area of the pelvic brim increases by 8% for every 1cm of joint separation.
The technique involves abducting the thighs to 80 degrees (but no further). A 2cm skin incision is made over the mons. With an index finger in the vagina displacing the urethra, the scalpel is inserted in the midline of the mons at the junction of the upper and middle thirds of the symphysis. If difficulty is experienced finding the ligament, a needle can be placed first. The blade is inserted until it impinges on the vaginal epithelium as determined by the finger in the vagina. Using the upper symphysis as a fulcrum, the knife is rotated, cutting the lower 2/3rds of the symphysis. The knife is then turned 180 degrees and the upper third of the symphyseal ligament is transected. Separation thus obtained is between 2 and 3cm -- the width of a thumb.
Following symphyseal separation, the bladder must be drained for five days. The patient is kept in bed on her side for three days. Sometimes the knees are tied together to enforce this position. On the fourth day the patient may sit in bed and on the fifth day walk. Results in terms of maternal recovery are uniformly excellent with return of full ambulation and pelvic stability.
The major risk is to maternal soft tissues including the bladder and urethra. As with many techniques, the more experience one obtains with procedure, the more quickly it can be performed and the lower the complication rate. Hartfield published a detailed description of symphysiotomy in 1973 in order to remind obstetricians that such a procedure exists. Although not advocating it in developed countries as a first step, he does state that it can be effected very quickly and may in some instances save a fetus' life when all other measures to resolve a shoulder dystocia have been exhausted. As he says in a second article he published on the subject in 1986,
The risk of maternal soft tissue trauma has to be weighed against the almost certain loss of the baby if other methods of vaginal delivery are proving unsuccessful.
All-fours maneuver
In 1976, Ina May Gaskin, a midwife, described a maneuver for the resolution of shoulder dystocia that involves placing the gravid mother on her hands and knees. Bruner (1998) used this procedure in 82 deliveries complicated by shoulder dystocia and was able to resolve the dystocia in 68 cases (82%) with this maneuver alone. The average time needed to move the mother into this position and to complete delivery was reported to be 2-3 minutes. Unfortunately, there was no detailed description of fetal and maternal outcome in this report. Also, reports about this procedure have generally been in the midwifery literature, involving a patient population less likely to have epidural anesthesia and thus more likely to be fully mobile.
It may be that the "all-fours maneuver" is merely another means of changing the angle of the symphysis in relation to the stuck shoulder, akin to McRoberts maneuver. Since the all-fours maneuver involves a gravid woman at the end of her pregnancy, exhausted by a long labor, often with an epidural in place, being moved quickly out of her delivery position onto all fours on her bed or on the floor, the practicality of this maneuver for a general obstetrical population is open to question. Unless more data is presented as to its efficacy and utility, it cannot be considered a standard procedure for the resolution of shoulder dystocia.
Are any particular maneuvers better than the shoulder dystocia resolution maneuvers?
This is not at all clear. There have been multiple reports by different authors claiming various degrees of success with each of the shoulder dystocia resolution maneuvers.
Leung in 2011 evaluated delivery methods in 205 cases of shoulder dystocia. He found that following the failure of McRoberts maneuver the subsequent application of rotational methods or of posterior arm delivery has similarly high rates of success although the former may be associated with less fetal injury. The rate of either brachial plexus injury or humoral fracture with rotational methods was 4.4% vs. 21% with delivery of the posterior arm. Leung et al suggest that delivery of the posterior arm is less safe than rotational methods.
Also, Leung’s success rate with McRobert’s maneuver alone in his largely Chinese patient population was only 25% as compared to multiple American studies showing the success rate with this maneuver to be in the 40% range.
On the other hand, Hoffman (2011) reviewed 132,098 deliveries in which there were 2018 shoulder dystocias for a rate of 1.5%. One hundred one of these--5.2%--resulted in a neonatal injury. Delivery of the posterior shoulder was associated with the highest rate of successful delivery compared to all other maneuvers with no difference in the rates of damage to the newborn. Hoffman recommends that the clinician move to delivery of the posterior shoulder if McRoberts maneuver and suprapubic pressure prove unsuccessful in the management of shoulder dystocia. The need for additional maneuvers was associated with higher rates of neonatal injury.
Finally, Spain (2015), in a study of 231 women who experienced a shoulder dystocia,
found that individual maneuvers were not associated with composite morbidity, neonatal injury, or neonatal depression after adjusting for parity and duration of shoulder dystocia. His conclusion:
There was no association between shoulder dystocia maneuvers and neonatal morbidity after adjusting for duration, a surrogate for severity. Our results demonstrate that the clinician should utilize the maneuver most likely to result in successful delivery.
So what can be said about the efficacy of the various shoulder dystocia resolution maneuvers?
1. The various maneuvers have not been subjected to a randomized trial
2. No maneuver has been clearly shown to be superior to any other in terms of successfully resolving a shoulder dystocia or reducing the rate of newborn injury.
What are some of the recommended protocols for resolving shoulder dystocia?
Many authors have proposed various protocols of prescribed maneuvers for the resolution of shoulder dystocia. Most are similar with only minor variations.
When a shoulder dystocia is recognized, it is generally agreed that McRoberts maneuver and suprapubic pressure should be implemented rapidly and simultaneously. These by themselves will resolve more than half of all shoulder dystocias. If the shoulder dystocia persists, other maneuvers can be performed in any order. These include the Wood's screw or Rubins maneuver in either the clockwise or counter clockwise direction, attempting to deliver the posterior arm, and, in extremis, consideration of such techniques as the Zavanelli maneuver or symphysiotomy.
ACOG, in its bulletin on shoulder dystocia (2002, reaffirmed 2015), proposed the following sequence of maneuvers for reducing a shoulder dystocia:
1) McRobert’s maneuver and suprapubic pressure
2) Episiotomy—controversial
3) Rotational maneuvers
4) Delivery of posterior arm
Harris in a 1984 paper recommended a similar protocol:
1) McRoberts maneuver.
2) Suprapubic pressure.
3) Large mediolateral episiotomy if above steps fail.
4) Wood's screw maneuver.
5) Attempt to free posterior arm.
Gherman (1998) discussed the protocol for managing shoulder dystocia utilized at that time at the University of Southern California:
McRoberts maneuver
Suprapubic pressure
Procto-episiotomy
Wood's corkscrew maneuver
Posterior arm extraction.
Zavanelli maneuver or symphysiotomy if all else fails.
McFarland (1996) reported that the use of two maneuvers alone --McRoberts and suprapubic pressure -- resulted in the resolution of 58% of 276 cases of shoulder dystocia in his series. He found that the addition of the Wood's Screw maneuver and delivery of the posterior arm were sufficient to resolve the shoulder dystocia in all remaining cases. He also found that there was a direct correlation between the rate of brachial plexus injury and the number of maneuvers employed to resolve the shoulder dystocia. A second correlation he found was that as the fetal weight increased, the number of maneuvers required to resolve shoulder dystocias increased.
Sentilhes (2016) discussed the guidelines for shoulder dystocia resolution from the French College of Gynecologists and Obstetricians (CNGOF)
Ask for help.
Perform McRoberts maneuver with or without suprapubic pressure.
Apply traction along the umbilical-coccygeal axis
Do either reverse Woods corkscrew maneuver or deliver posterior arm
Perform an episiotomy if one has not yet been performed one
Repeat maneuvers
Go to third line maneuvers.
Sentilhes adds two notes:
1. The available data do not allow us to conclude that any one of these maneuvers is superior to any other.
2. The performance of these obstetrical maneuvers for treating shoulder dystocia does not routinely require an episiotomy.
As has been shown, different authors recommend different combinations of maneuvers in attempting to resolve shoulder dystocias. But what every author emphasizes, and what the ACOG bulletin stresses, is that the most important aspect of resolving a shoulder dystocia is for the obstetrician to have a clear-cut, well thought-out sequence of maneuvers already in mind when a shoulder dystocia is encountered. The general consensus is that the best results in resolving shoulder dystocias are obtained when an obstetrician:
(1) Recognizes the shoulder dystocia
(2) Knows the different maneuvers involved in attempting to resolve shoulder dystocia
(3) Implements them in a carefully controlled, calm, and organized fashion.
Is all brachial plexus injury caused by shoulder dystocia and/or “excessive” physician traction?
In his 2002 paper, Pecorari states the following:
Unfortunately for obstetricians and midwives, in court Erb palsy has been causally connected with shoulder dystocia and errors in management, although it is not always true. Perhaps the lack of an obvious explanation has contributed to the identification of the birth attendant as a handy scapegoat.
When there is a permanent brachial plexus injury following shoulder dystocia, responsibility for this injury is often presumed to lie with the obstetrician who supposedly did not foresee that a shoulder dystocia was likely to occur or mishandled it when it did. Yet a review of the literature does not substantiate such assumptions.
Gherman in his 1998 paper summarizes the refutation to these claims:
We feel that some cases of brachial plexus injury are unavoidable events. Recent reports have noted that brachial plexus palsies occur:
(1) In the absence of characteristic risk factors
(2) In the absence of shoulder dystocia
(3) In the posterior arm of infants whose anterior shoulder was impacted behind the symphysis pubis
(4) In vertex-presenting fetuses delivered by atraumatic Cesarean section
(5) Without apparent relationship to the type or number of maneuvers used to disimpact the fetal shoulder
(6) In association with other peripheral nerve injuries
(7) With electromyelographic evidence of muscular denervation during the immediate postpartum period [subsequently disproven].
Jennett commented in a similar vein in 1997:
Evidence continues to accumulate that renders a univariate theory of the causation of brachial plexus injury untenable . . . Intrauterine maladaption is responsible for some instances of brachial plexus injury.
He notes that in his series of deliveries from 1977 to 1990, 22 of 39 (56%) brachial plexus injuries were not associated with shoulder dystocia. In that same paper he quotes Pearl (1993) and Gimovsky (1995), both of whom reported brachial plexus injuries in babies delivered from the occiput posterior position without shoulder dystocias. He further cites Walle (1993) who observed in his patient population that 1/3rd of 175 shoulder dystocias involved the posterior shoulder.
There are many other similar reports:
Hardy (1981) reported 36 infants with brachial plexus injuries of whom only 10 had shoulder dystocia noted at birth.
Gilbert (1990) initially published a study of 1000 infants with brachial plexus injury in which 39% did not have shoulder dystocia at delivery. In a supplementary article in 1999, he reported that 47% of babies with brachial plexus injury in his now larger series experienced deliveries in which no shoulder dystocia was noted. Even among macrosomic fetuses in this series, 26% of brachial plexus injuries occurred in the absence of shoulder dystocia.
Gonik (1991) reported that 71% of all injured infants in his series were the product of deliveries without recognized shoulder dystocia.
Sandmire (1996) reported 17 babies in his series of 36 with brachial plexus injuries whose deliveries did not involve shoulder dystocia. This article included his personal review of the literature concerning brachial plexus injury (BPI) with and without shoulder dystocia:
|
Author |
# of
deliveries greater than 4,500 grams |
BPI with shoulder dystocia |
BPI without shoulder dystocia |
| Lipscomb
(1995) |
157 |
7/12 |
5/12
|
| Mentocoglou
(1992) |
589 |
5/9 |
4/9
|
| Sandmire
(1996) |
547 |
9/19
|
10/19
|
| |
|
|
|
| TOTALS
|
1,727 |
34/69 (49%) |
35/69 (51%) |
As can be seen, 51% of brachial plexus injuries in over 1727 deliveries of macrosomic babies did not involve shoulder dystocias.
Gram (1997) noted that only 8 babies had shoulder dystocia deliveries in a group of 15 who experienced brachial plexus injury. In the other seven cases, there had been no birth trauma.
Hillard's series of babies with Erb palsy (1997) described 15 of 51 babies who had not experienced shoulder dystocia during delivery.
Torki (2012) presented a series of eight cases from the University of Southern California where there was no shoulder dystocia yet there was severe brachial plexus palsy. While the authors do not state whether the injuries to these neonates were permanent, they were severe enough that the infants had to be admitted to the neonatal intensive care unit.
El-Sayed (2013) studied obstetrical brachial plexus palsy over the last 18 years in his clinic in Saudi Arabia. Of 751 cases 33% resulted from routine deliveries. They conclude that the normal forces of labor and delivery can lead to obstetric brachial plexus palsy.
Iskender (2014) reviewed 44,092 vaginal deliveries from his hospital in Ankara Turkey between 2009 and 2013. Among six neonates with permanent brachial plexus injury, five of them had not experienced shoulder dystocia.
Zuarez-Easton et. al. (2015) evaluated 83,806 deliveries from their hospital in Israel. There were 144 cases of brachial plexus injury for a rate of 1.7 per thousand. In 41 of 144 cases (28.9%) there was no documented shoulder dystocia—and this in an era and facility where shoulder dystocia simulation and documentation were both being practiced.
Gurewitsch and Allen (2006), while reporting that 90% of their persistent brachial plexus injury cases were related to documented shoulder dystocia, show that 10% of permanent injuries either had no documentation of or no occurrence of shoulder dystocia. The object of their paper was to ascertain whether brachial plexus palsy that occurs without shoulder dystocia represented a traction injury during unrecognized shoulder dystocia or a natural phenomenon with a different mechanism of injury. They compared risk factors and outcomes between shoulder dystocia-associated and non-shoulder dystocia-associated brachial plexus palsy cases using two datasets, one from Johns Hopkins Hospital from 1993 to 2004 and the other from a series of litigated cases.
In 11.5 years at Johns Hopkins Hospital there were 135 brachial plexus palsies among 23,273 deliveries. There were 206 cases in the litigated series. Of these, in 1 of 8 in the hospital group and in 6 of 206 of the litigated cases, no shoulder dystocia was documented. Gurewitsch and Allen state in the last paragraph of their paper that
The current investigation supports that intrauterine and intrapartum phenomena can contribute to the mechanism of birth -related brachial plexus palsy.
They conclude that non-shoulder dystocia brachial plexus palsy is real though uncommon and likely occurs by modes of injury mechanically distinct from shoulder dystocia brachial plexus palsy.
Finally, the definitive 2014 ACOG report on Neonatal Brachial Plexus Palsy states
“Neither high-quality nor consistent data exist to suggest that neonatal brachial plexus palsy can be caused only by a specific amount of applied force beyond that typically used by healthcare providers during any delivery. Instead, available data suggests that the occurrence of a neonatal brachial plexus palsy is a complex event, dependent not only on the forces applied at the moment of delivery, but also on the constellation of forces…. that have been acting on the fetus during the labor and delivery process as well as individual fetal tissue characteristics.
The report goes on to say that
“Recent multidisciplinary research now stresses that the existence of neonatal brachial plexus palsy following birth does not a priori indicate that exogenous forces are the sole cause of this (i.e. brachial plexus nerve) injury.”
What does cause brachial plexus injuries?
In court, the standard explanation for a brachial plexus injury is that it results from excessive downward traction by the obstetrician on the fetal head during an attempt to resolve a shoulder dystocia. This supposedly overstretches the brachial plexus thus injuring it.
Yet significant endogenous forces are generated through the natural physical events of labor. The forces of both uterine contractions and maternal pushing move a fetus from the uterus through the birth canal and out of the maternal pelvis. Stretching of the brachial plexus occurs during this process, as shown by both computer simulations and physical models. The stretch results from differential motion between the fetal head and shoulders after some element of the maternal anatomy—usually the symphysis pubis--retards the progression of the fetal shoulders.
The tractor-trailer theory
Sandmire (2000) was among the first to describe how the forces of labor and maternal pushing could result in brachial plexus injuries unrelated to physician traction at delivery. Sandmire studied what happens to the various parts of the fetus during uterine contractions and maternal pushing. He noted that the forces of contractions and maternal pushing act on the long axis of the fetus. If the fetus's anterior shoulder were to get stuck behind the maternal pubic bone and continued pressure was applied to the long axis of the fetus, the baby's brachial plexus would undergo considerable stretching.
This may be compared to what happens when a tractor-trailer truck gets stuck under a low overpass. While the cab may pass under the bridge, the trailer -- taller than the cab--may get caught on the overpass and will be unable to emerge no matter how hard and frequently the driver in the cab “guns” the engine. This process will, however, generate large stretching forces in the connectors between the tractor and the trailer. Sandmire suggests that an equivalent force acts upon a baby's brachial plexus during shoulder dystocia deliveries.
Evidence of such stretching is clinically observed with the “turtle sign”. This phenomenon is encountered at the moment of delivery when, after the head emerges, it is often pulled back against the perineum with significant, forceful recoil. This “recoil” is caused by the spring-like action of the markedly stretched neck skin, muscle, and brachial plexus tissue during the mother’s last push to deliver her baby’s head. During this last thrust, the shoulders remain unmoved--restrained by the mother’s symphysis pubis—while the head emerges several centimeters out from the vagina orifice, greatly stretching the brachial plexus nerves.
Stretching of the brachial plexus nerves does not only occur during the last push of labor. It likely takes place through much of the late second stage when the fetus’s head is low in the pelvis yet the fetal body is prevented from moving lower in the birth canal because of obstruction of its shoulders on the mother’s pubis.
Forces involved in shoulder dystocia deliveries
In 1991 Robert Allen published an article wherein it was suggested that 100lbs of force was the amount necessary to injure a brachial plexus, a claim which he has subsequently made in other forums (Allen, 1999). Yet this data was based on the measurement of a single baby who experienced a temporary brachial plexus at birth. Moreover Gonik—who was the co-author with Allen on the 1991 paper—subsequently showed (2000) that maternal endogenous forces—those of maternal contractions and pushing—resulted in several fold the force level applied by a delivering physician.
Deering (2011) also published an article in which he expressed skepticism about the “rather arbitrary cutoff of 100 N [that] is generally accepted as the maximum force that should be applied during a delivery”. He reports how studies by Croft in the United Kingdom show that forces exceeded 100 Newton in more than two thirds of shoulder dystocia delivery simulations. He concludes that either simulations do not correctly represent what happens at regular deliveries or the 100 Newton number is incorrect. If not, than a very large percentage of babies would suffer brachial plexus injury at birth.
Another point—made by Allen (2005) —is that some fetuses may be more predisposed than others to brachial plexus injury merely because of “biologic variation” That is, some fetuses may have structurally weaker nerve tissue than others, be born in a position that more significantly stretches this tissue, or at the time of delivery--due to hypoxia or other causes—have less protection from surrounding intact muscles than do other neonates.
There is another force at birth that operates on the fetal brachial plexus: compression. Gonik (2003) has shown that during a shoulder dystocia the fetus’s shoulder and neck are pushed against the maternal symphysis pubis with such large pressures that the neonate’s brachial plexus may be injured. This methodology of injury is contested by some who claim that compression cannot cause brachial plexus injuries. Yet we know from many other examples in obstetrics and gynecology that compression injuries can cause permanent nerve damage: forceps damage to facial nerves, self-retaining retractor damage to obturator and femoral nerves, etc.
The 2014 ACOG report “Neonatal Brachial Plexus Policy” discusses the forces potentially involved in brachial plexus injuries in detail. They conclude:
Neonatal brachial plexus palsy can occur even when axial traction is properly applied. The occurrence of this injury does not automatically indicate that the practitioner applied forces or maneuvers that caused the nerve injury. The forces of uterine contractions and maternal pushing alone are probably sufficient to cause excessive traction on the brachial plexus. Many cases of brachial plexus injury occur independently of shoulder dystocia or excessive force by the provider. A substantial portion of neonatal brachial plexus palsy cases are not associated with antecedent shoulder dystocia. These injuries occur during cesarean section deliveries.
Posterior shoulder
Most brachial plexus injuries occur to a baby's right arm (60%). This is because babies most commonly "present" into the mother's pelvis in the left occiput anterior position (LOA). The LOA position is when the back of the baby's head -- the occiput -- points towards the mother's left arm while the fetal face is oriented towards the mother's right buttock. In this fetal position the baby's right arm will be anterior -- and thus more likely to get caught under the mother's pubic bone. But many brachial plexus injuries have also been reported in the posterior shoulder. It is thought that in these cases the posterior shoulder gets caught on and restrained by the sacral promontory while the remainder of the baby is being pushed forward by the mother's expulsive efforts and uterine contractions.
Brachial plexus injuries following Cesarean section
Reports of brachial plexus injury in the absence of shoulder dystocia are subject to the criticism that perhaps shoulder dystocias were under-reported or that "excess' traction might have been placed on the baby's head during the course of a routine delivery. But reports of brachial plexus injury following Cesarean section are less subject to criticism. There are many such reports in the literature:
Ecker (1997): Two infants born by Cesarean section who sustained brachial plexus injuries, one of a nondiabetic mother, the other of a diabetic mother.
Hardy (1981): Two infants born in vertex position at Cesarean section who sustained brachial plexus injuries.
McFarland (1986): Four patients delivered by Cesarean section who experienced brachial plexus injuries.
Graham (1997): Report of an Erb palsy from cesarean section.
Gilbert (1999): Evaluated data on all brachial plexus injuries from California in the years 1994 to 1995. Of the 1,094,298 babies born in those two years there were 1,611 brachial plexus injuries reported (0.15%). Of these, 60 of the injured babies were born via cesarean section.
The above is only a brief sample of many such papers.
Arguments by claimants that brachial plexus injury is always or almost always caused by physician traction
A study by the Swedish obstetrician Mollberg (2008) is often quoted as demonstrating that brachial plexus injury is correlated with an increase in physician traction during delivery. However a closer look at her data reveals an interesting feature: Of the 18 cases of permanent neonatal brachial plexus palsy she describes, fundal pressure was used in the delivery of 17 of them. Thus, as the 2014 ACOG publication on Neonatal Brachial Plexus Palsy states,
Despite the conclusions by Mollberg and colleagues that forceful downward traction was causative, it is not possible to separate out the effect of increased traction from the effect of increased expulsive force induced by the application of fundal pressure.
Others who claim that physician expulsive forces are the only cause of permanent brachial plexus often cite cadaveric studies in an attempt to show that the amount of force necessary to sever the nerves of the brachial plexus are sometimes exceeded by physicians during deliveries. But most of these quoted studies are between 50 and 100 years old: Sever 1916, Adson 1922, Morris 1955. The author of a more recent study (Metaizeau (1979) says that his data is not a good means of assessing the forces in a live baby during a delivery complicated by shoulder dystocia. As the ACOG 2014 publication suggests, such anatomic studies are “quite crude” by today’s standard of biomechanics. Furthermore, “they do not provide a complete picture of how and why neonatal brachial plexus palsy may occur during delivery”. The ACOG publication concludes this section of the report by stating:
It is inappropriate to conclude that lateral bending is the only cause of the injury on the basis of these early studies when similar research has not examined other mechanisms.”
The ACOG 2014 publication from the Neonatal Brachial Plexus Palsy Task Force
This task force, convened by James Breeden MD, then President of the American College of Obstetricians and Gynecologist (2012-2013), and Albert Strunk JD, MD, Deputy Executive Vice President of the American College, was established to provide “a well-researched, informative, objective, and dispassionate presentation of the existing state of knowledge” concerning neonatal brachial plexus palsy. The members who made up the panel are among the most knowledgeable experts in the world on shoulder dystocia and brachial plexus injury, having between them published scores of articles on this topic. The following are some conclusions from this ACOG report:
The existence of neonatal brachial plexus palsy following birth does not a priori indicate that exogenous forces are the sole cause of this injury p. ix
Neonatal brachial plexus palsy also has been shown to occur entirely unrelated to traction, with studies demonstrating cases of both transient and persistent neonatal brachial plexus palsy in fetuses delivered vaginally without clinically evident shoulder dystocia or fetuses delivered by cesarean section without shoulder dystocia. P. 17
Traction applied in the plane of the fetal cervical-thoracic spine is typically along a vector estimated to be 25 – 45° below the horizontal plane when the woman in labor is in lithotomy position. P. 24
Uterine contractions result in a compression force to the fetus that acts to move the entire fetus down the birth canal. If, during this movement, a structure obstructs a body part while another body segment continues moving forward, the difference in motion can result in either a pulling force on the tissues that connect the two regions or a bending force on a rigid, bony body part. P. 24.
Maternal forces, the combination of uterine contractions and maternal pushing, are likely to be at least 140 – 160 N during the second stage of labor when intrauterine pressure of 120 mmHg is common….. Thus it can be anticipated that maternal forces during the second stage of labor will reach at least 140 N with an average size fetus. P. 25
If a shoulder is restrained, maternal forces will continue to move the head and neck forward, widening the angle between the neck and the shoulder and causing traction on the brachial plexus. P. 27
Gherman and colleagues hypothesized that several mechanisms of injury may occur, depending on the characteristics of the fetus and the delivery, for example:
Continued movement of the head following impaction of the anterior shoulder behind the symphysis pubis or impaction of the posterior shoulder on the sacral promontory.
Normal downward traction applied by the physician in the presence of observed shoulder dystocia.
Compression of the brachial plexus against the symphysis pubis.
Abnormal intrauterine pressure arising from uterine anomalies or uterine hypertonicity. P. 28
No published clinical or experimental data exist to support the contention that the presence of persistent (as compared to transient) neonatal brachial plexus palsy implies the application of excessive force by the birth attendant. P. 28
In summarizing the pathophysiology and causation of neonatal brachial plexus injury, the ACOG report states:
Neither high-quality nor consistent data exist to suggest that neonatal brachial plexus palsy can be caused only by a specific amount of applied force beyond that typically used by healthcare providers and experienced during a delivery without neonatal brachial plexus palsy. Instead, much of the data suggest that the occurrence of neonatal brachial plexus palsy is a complex event, dependent not only on the forces applied at the moment of delivery, but also on the constellation of forces (e.g., vector and rate of application) that have been acting on the fetus during the labor and delivery process, as well as individual fetal tissue characteristics (e.g., in situ strain and acid-base balance). P. 37
As has been shown, there is much evidence that not all instances of brachial plexus injury are due to the actions of a physician during a shoulder dystocia delivery. Thus the automatic assignment of responsibility to an obstetrician or midwife for a brachial plexus injury is inappropriate and is not supported by the obstetrical literature.
Shoulder Dystocia Training
A shoulder dystocia drill is a practice run-through by a labor and delivery unit of a mock shoulder dystocia delivery. Because shoulder dystocia—like most severe obstetrical emergencies—occurs too infrequently for skill to be developed in handling it properly just by routine work on the Labor and Delivery floor, training with shoulder dystocia drills has been suggested both as a practice protocol and as a teaching technique for all members of the obstetrical team. Some authors have stated that it is the obligation of every delivery unit and every obstetrician to participate in routine shoulder dystocia drills as part of obstetrical readiness. There are now several excellent videos on line –including one produced by the American College of Obstetricians and Gynecologists (ACOG) -- AVL 103 -- that describe and visually demonstrate model shoulder dystocia drills.
There have been multiple reports in the recent obstetrical literature by units that have instituted such drills, with surprisingly varied results. One has to be aware when reading this literature as to whether the improvements claimed are from real shoulder dystocia deliveries or are only improvements in drill performance.
Draycott and Crofts from England were among the first to implement and study the results of shoulder dystocia simulation. In 2005 they develop a unique manikin for training and investigated its effectiveness in improving performance of physicians and students in initiating the correct steps for shoulder dystocia resolution. They found that the management of shoulder dystocia improved following training with the manikin. There was a reduction in both the head-to-body delivery duration and in maximum applied delivery forces. They specifically noted that after training no subject applied of delivery force greater than 100 Newtons.
Draycott (2008) then took the next step: He compared the management of shoulder dystocia and neonatal outcomes before and after introduction of his shoulder dystocia training program in live births at a hospital in southwest England. He was able to show for the first time with real deliveries that there was a significant reduction in neonatal injury from shoulder dystocia--9.3% compared to 2.3%--after the introduction of a shoulder dystocia training program for all maternity staff. Subsequently Deering (2011) demonstrated similar findings in the United States
Grobman (2011) studied the results of the implementation of a shoulder dystocia protocol focused on total team response. His group measured the results of shoulder dystocia deliveries in three six-month periods, one before, one during, and one after the protocol was established. Complete and consistent documentation increased from 14% to 92% while the incidence of brachial plexus palsy fell from 10.1% to 4.0% and finally to 2.6%. Thus study confirmed the utility of a shoulder dystocia training program for labor and delivery units.
Inglis (2011) implemented a training program for shoulder dystocia for his entire maternity staff. His group found that the overall incidence of obstetrical brachial plexus injury from vaginally deliveries decreased from 0.4% (pretraining) to 0.14% (post training). Interestingly, after shoulder dystocia training there was a decrease in the use of McRoberts maneuver and an increase in the use of posterior arm delivery and the Rubins maneuver.
But not all studies on shoulder dystocia simulation training have shown the same successful results described above:
Walsh (2011) compared two time periods—1994-1998 and 2004-2008. The second time period was after there had been a specific staff training program in the management of shoulder dystocia. He found that the incidence of brachial plexus injury remained unchanged: 1.5 per 1000 deliveries in the earlier group and 1.7 per 1000 deliveries after the training program had been implemented.
Comeau (2014) reported on a group of 17 obstetrical residents who were offered a training program in the documentation of shoulder dystocia deliveries. Assessed as a group, there were no differences in the completeness of documentation before and after the simulation session.
Kim (2016) initiated a program of mandatory shoulder dystocia simulation training for obstetrical providers at the University of Minnesota in Minneapolis. While this program resulted in an increase in the identification of shoulder dystocia events, there was no decrease in adverse maternal or neonatal outcomes. He concluded that provider training alone does not impact adverse maternal and neonatal outcomes.
Although practicing and preparing for any emergency is always a good idea, it is not clear whether a formalized drill performed at regular intervals is necessary to provide good care. What is necessary, however, is that obstetricians, obstetrical nurses, and everyone involved with deliveries know that any vaginal delivery can suddenly turn into a shoulder dystocia emergency. They therefore must be aware of and able to perform the steps necessary to resolve this emergency in an orderly, efficient manner.
Documentation
Careful documentation of instances of shoulder dystocia and their resolution is extremely important for two reasons:
1) Obstetricians want to learn as much as possible from instances of shoulder dystocia in order to develop the best techniques for dealing with them.
2) An injury following a shoulder dystocia delivery often results in medical-legal actions. Accurate, contemporaneous documentation of what the provider did and what his or her thought process was will be invaluable in defending the care that was given.
Acker (1991) described what careful documentation of a shoulder dystocia delivery should include:
1) Exact times of events.
2) Description of the maneuvers used.
3) Estimation of the traction forces exerted.
The note must be legible and must be written or dictated shortly after the events so that it is a contemporaneous medical progress note. Acker also recommends that the note have a specific form. This would include comments on:
1) Delivery time both for head and body (the nurse should record this).
2) Episiotomy description and timing.
3) Whether or not anesthesia was present when the shoulder dystocia was recognized and any additional anesthesia given.
4) Nasopharyngeal suction.
5) Initial traction before shoulder dystocia is recognized, documenting force and duration.
6) Maneuvers used, listing them in the order employed.
7) The force used described in comparative terms such as average, maximal, etc.
8) Duration of maneuvers -- have the nurses know to record this.
9) Personnel -- identify all present.
10) Estimated fetal weight and the actual birth weight.
Experience has shown that the best defense in a medical liability action, whether involving shoulder dystocia or any other situation, is thoughtful, articulate, timely documentation of each decision made in the course of treatment.
Yet how one teaches proper documentation and tries to assure that it is done correctly in practice has proven problematic.
Crofts (2008) set up a program in which midwives and junior and senior obstetricians in six hospitals in southwest England were trained in all aspects of shoulder dystocia care. Part of the training included a 40 minute practical workshop on documentation of shoulder dystocia deliveries. At the end of a simulation each participant was asked to document what they had done. In a total of 110 participants, only 56% documented the head-to-body delivery interval with 56% of these overestimating the time by more than one minute. The force used during the simulation was documented by 70.9% of participants. Documentation of force was more likely if a preformatted medical record sheet was provided.
Croft’s conclusion:
1. Maneuvers performed were well documented.
2. Head-to-body delivery intervals and force applied were not documented accurately in the majority of simulated deliveries.
3.Use of a preformatted sheet appears to improve completeness but not accuracy of documentation.
Moragianni (2013) reviewed the charts of 100 vaginal deliveries complicated by shoulder dystocia before and 81 after the implementation of a standardized delivery form. Charts that included the standardized delivery form were more likely to describe important parameters about the delivery. He concluded that inclusion of a standardized form in the delivery record improves the rate of comprehensive documentation of shoulder dystocia deliveries.
Comeau (2014), on the other hand, trained 18 residents in shoulder dystocia documentation after which he tested them. The results: there was no difference in the quality of reporting on shoulder dystocia deliveries compared to that prior to training.
Accurate documentation of events in a shoulder dystocia delivery is important for both medical and medical-legal reasons. This is a skill that has to be taught. There are tools—such as delivery note templates—that can increase the accuracy and compliance with such documentation—but such training efforts have not been uniformly successful.
Conclusions
A review of the literature on shoulder dystocia reveals the following:
1. Despite the use of ultrasound to attempt to estimate fetal weights, there is currently no way for obstetricians to determine with any degree of accuracy which babies will be macrosomic or will experience shoulder dystocia at delivery. New work on shoulder dystocia prediction algorithms may change this existing limitation in obstetric practice.
2. The various strategies proposed to attempt to reduce the number of shoulder dystocia deliveries and brachial plexus injuries would result in:
a. The performance of hundreds or thousands of cesarean sections to prevent a single case of permanent brachial plexus injury
b. The potential medical complications from such interventions
c. The economic costs of such interventions
3. Although there are various techniques for resolving shoulder dystocias, these will not totally eliminate the incidence of brachial plexus palsies and other birth injuries.
4. Brachial plexus injuries may be caused by multiple factors related to the physiology of labor and delivery.
5. No published clinical or experimental data exist to support the contention that the presence of persistent neonatal brachial plexus palsy could only be caused by the application of excessive force by the birth attendant.
BibliographyAbrams
B, Selvin, S. Maternal weight gain pattern and birth weight.
Obstet Gynecol 1995
(1);86;163-169.
Abrams B, Carmichael S,
Selvin S. Factors associated with the pattern of maternal
weight
gain during pregnancy. Obstet Gynecol 1995 (2);86:170-176.
Acker D., Sachs B., Friedman
E. Risk factors for shoulder dystocia. Obstet Gynecol
1985;66:762-768.
Acker D., Sachs B, Friedman
E. Risk factors for shoulder dystocia in the average weight
infant. Obstet Gynecol 1986; 67:614-61.
Acker D., Gregory K, Sachs B,
Friedman E. Risk factors for Erb-Duchenne Palsy. Obstet
Gynecol 1988;71:389-392.
Acker D. A shoulder dystocia
intervention form. Obstet Gynecol 1991; 78:150-151.
Ahern J., Goodlin R. Cesarean
section in the massively obese. Obstet Gynecol, 1978;
51:509-510.
Al-Qattan MM, al-Kharfy TM.
Obstetric brachial plexus injury in subsequent deliveries. Ann
Plas Surg 1996;37:545-8.
Allen R, Sorab J, Gonik B.
Risk factors for shoulder dystocia: An engineering study of
clinician-applied forces. Obstet Gynecol. 1991;77:352-355.
Allen RH, Bankoski BR,
Burtzin CA, et al. Comparing clinician-applied loads for
routine ,
difficult, and shoulder dystocia deliveries. Am J Obstet
Gynecol. 1994;171:1621-1627.
Al-Najashi S, Al-Suleiman S,
El-Yahia A, Rahman M, Rahman, J. Shoulder dystocia--A clinical
study of 56 cases. Aus NZ J Obstet Gynecol, 1989; 29:129-132.
American College of
Obstetricians and Gynecologists. Fetal Macrosomia. Washington
DC:
ACOG, 2000. Practice Bulletin No. 22.
American College of
Obstetricians and Gynecologists. Shoulder Dystocia.
Washington, D.C.:
ACOG, 1997.
American College of
Obstetricians and Gynecologists. Practice Bulletin Number 40:
Shoulder
Dystocia. Washington, D.C.: November 2002.
Bahar AM. Risk factors and
fetal outcome in cases of shoulder dystocia compared with
normal
deliveries of a similar birthweight. British Journal of
Obstetrics and Gynecology: 1996;
103:868-872.
Barden TP, Knowles, HC Jr.
Diagnosis of diabetes in pregnancy. Clinical Obstetrics and
Gynecology, Vol 24, No 1, March 1981.
Baskett TF, Allen AC.
Perinatal Implications of Shoulder Dystocia. Obstet Gynecol:
1995;
86:14-17.
Beall MH, Spong C, McKay J,
Ross MG. Objective definition of shoulder dystocia: A
prospective evaluation. Am J Obstet Gynecol 1998;179:934-7.
Benedetti TJ, Gabbe SG.
Shoulder dystocia: A complication of fetal macrosomia and
prolonged second stage of labor with midpelvic delivery.
Obstet Gynecol 1978:52:526-9.
Benson CB, Doubilet PM,
Saltzman DH, Greene MF, Jones TB. Femur length/abdominal
circumference ratio: Poor predictor of macrosomic fetuses in
diabetic mothers. Journal of
Ultrasound in Medicine 1986;5:141-4.
Benson CB, Doubilet PM,
Saltzman DH. Sonographic determination of fetal weights in
diabetic
pregnancies. Am J Obstet Gynecol 1987;156:441-4.
Berard J, Dufour P, Vinatier
D, et al. Fetal macrosomia: risk factors and outcome. A
study of the outcome concerning 100 cases >4500 g. Eur J
Obstet Gynecol. 1998;77:51-59.
Blickstein I, Ben-Arie A,
Hagay ZJ. Antepartum risks of shoulder dystocia and brachial
plexus injury for infants weighing 4,200 g or more. Gynecol
Obstet Invest. 1998;45:77-80.
Bofill JA, Rust OA, Devidas
M, et al. Shoulder dystocia and operative vaginal delivery. J
Maternal Fetal Med. 1997;6:220-224.
Boyd ME, Usher RH, McLean FH.
Fetal macrosomia: Prediction, risks, proposed management.
Obstet Gynecol. 1983;61:715-722.
Bradley RJ, Nicolaides KH,
Brudenell JM. Are all infants of diabetic mothers "macrosomic"?
BMJ 1988;297:1583-4.
Bruner JP, Drummond SB,
Meenan AL, et al. All-fours maneuver for reducing shoulder
dystocia
during labor. J Reprod Med. 1998;43:439-443.
Bryant DR, Leonardi MR,
Landwehr JB, Bottoms SF. Limited usefulness of fetal weight in
predicting neonatal brachial plexus injury. Am J Obstet
Gynecol 1998;179:686-9.
Buhimschi CS, Buhimschi IA,
Malinow IA, et al. Use of McRoberts' position during delivery
and increase in pushing efficiency. Lancet. 2001;358:470-471.
Casey, BM, Lucas, MJ,
Mcintire, DD, Leveno, KJ. Pregnancy outcomes in women with
gestational diabetes compared with the general obstetric
population. Obstet Gynecol, 1997;
90:869-873.
Chambrelent. Deux cases des
dystocie par volume exaggere des epaules. Rev Mens Gynec
Obstet de Bordeaux. 1902;2:88.
Chauhan SP, Cowan BD, Magann
EF, Bradford TH, Roberts WE, Morrison JC. Intrapartum
detection of a macrosomic fetus: Clinical versus 8 sonographic
models. Aust & NZ J Obstet
& Gynecol, 1995; 35:266.
Chauhan SP, Lutton PM, Bailey
KJ, Guerrieri JP, Morrison JC. Intrapartum clinical,
sonographic, and parous patients' estimates of newborn birth
weight. Obstet & Gynecol,
1992; 79:956-958.
Chervenak JL, Divon MY,
Hirsch J, GirzBA, Langer O. Macrosomia in the postdate
pregnancy:
Is routine ultrasonographic screening indicated? Am J Obstet
Gynecol 1989; 161:753-756.
Chez RA, Carlan S, Greenberg
SL, Spellacy WN. Fractured clavicle is an unavoidable event.
Am J Obstet Gynecol 1994;171:797-8.
Coen RW, Porreco R, Cousins
L, Sandler JA. Postpartum glycosylated hemoglobin levels in
mothers of large-for-gestational age infants. Am. J. Obstet.
Gynecol 1980; 136:380.
Cohen B, Penning S, Major C,
et al. Sonographic prediction of shoulder dystocia in infants
of diabetic mothers. Obstet Gynecol. 1996;88:10-13.
Cohen BF, Penning S, Ansley
D, et al. The incidence and severity of shoulder dystocia
correlates with a sonographic measurement of asymmetry in
patients with diabetes. Am J
Perinatol. 1999;16:197-201.
Cohen WR. Influence of the
duration of second stage labor on perinatal outcome and
puerperal morbidity. Obstet Gynecol 1977; 49:266-268.
Collins JH, Collins CL. What
is shoulder dystocia? J Reprod Med. 2001;46:148-149.
Combs CA, Singh NB, Khoury JC.
Elective induction versus spontaneous labor after
sonographic diagnosis of fetal macrosomia. Obstet Gynecol.
1993;81:492-496.
Conway Dl, Langer O. Elective
delivery of infants with macrosomia in diabetic women:
Reduced shoulder dystocia versus increased cesarean
deliveries. Am J Obstet Gynecol.
1998;178:922-925.
Coustan DR, Imarah J.
Prophylactic insulin treatment of gestation diabetes reduces
the
incidence of macrosomia, operative delivery, and birth trauma.
Am J Obstet Gynecol 1984;
150:836-42.
Dawes MG, Grudzinskas JG.
Patterns of maternal weight gain in pregnancy. British J
Obstet
Gynecol 1991;98:195-201.
de Chalain TMB, Clarke HM,
Curtis CG. Case report: Unilateral combined facial nerve and
brachial plexus palsies in a neonate following a midlevel
forceps delivery. Annals of
Plastic Surgery 1997;38:187-190.
Delpapa DH, Mueller-Heubach
E. Pregnancy outcome following ultrasound diagnosis of
macrosomia. Obstet Gynecol 1991;78:340-343.
Deter RL, Hadlock FP. Use of
ultrasound in the detection of macrosomia: A review. J Clin
Ultrasound 1985;13:519-524.
Dignam WJ. Difficulties in
delivery, including shoulder dystocia and malpresentations of
the fetus. Clinical Obstet Gynecol 1976;19:577-585.
Dornan KJ, Hansmann M,
Redford DHA, Wittmann BK. Fetal weight estimation by real-time
ultrasound measurement of biparietal and transverse trunk
diameter. Am J Obstet. Gynecol
1982;142:652-657.
Dyachenko A, Ciampi A, Fahey J, Mighty H, Oppenheimer L, Hamilton EF. Prediction of risk for shoulder dystocia with neonatal injury. Am J Obstet Gynecol. 2006 Jul 14; [Epub ahead of print]
Ecker JL, Greenberg JA,
Norwitz ER, Nadel AS, Repke JT. Birth weight as a predictor of
brachial plexus injury. Obstet Gynecol 1997;89:643-647.
Elliott, Lt Col JP, Garite TJ,
Freeman RK, McQuown DS, Patel JM. Ultrasonic prediction of
fetal macrosomia in diabetic patients. Obstet Gynecol
1982;60:159-162.
El Madany AA, Jallad KB, Radi
FA, El Hamdan H, O'Deh HM. Shoulder dystocia: anticipation
and outcome. Int J Gynecol Obstet, 1990;34:7-12.
Emerson, R. Obesity and its
association with the complications of pregnancy. Br Med J.
1962; 2:516-519.
Flamm BL. Tight nuchal cord
and shoulder dystocia: A potentially catastrophic combination.
Obstet Gynecol. 1999;94:853.
Friesen CD, Miller AM,
Rayburn WF. Influence of spontaneous or induced labor on
delivering
the macrosomic fetus. Am J Perinatol. 1995;12:63-66.
Geary M, McParland P, Johnson
H, et al. Shoulder dystocia: Is it predictable? Eur J
Obstet Gynecol Reprod Biol. 1995;62:15-18.
Gemer O, Bergman M, Segal S.
Labor abnormalities as a risk factor for shoulder dystocia.
Acta Obstet Gynecol Scand. 1999;78:735-736.
Gherman RB, Goodwin TM,
Souter I, Neumann K, Ouzounian JG, Paul Richard. The McRoberts'
maneuver for the alleviation of shoulder dystocia: How
successful is it?. Am J Obstet
Gynecol 1997;176:656-61.
Gherman RB, Ouzounian JG,
Incerpi MH, Goodwin TM. Symphyseal separation and transient
femoral neuropathy associated with the McRoberts' maneuver. Am
J Obstet Gynecol
1998;178:609-610.
Gherman RB, Ouzounian JG,
Goodwin, TM. Obstetric maneuvers for shoulder dystocia and
associated fetal morbidity. Am J Obstet Gynecol
1998;178:1126-30.
Gherman R. Reply to William
Spellacy's letter to the editor, "Erb palsy without shoulder
dystocia," 1998;179:561-2.
Gherman RB, Ouzounian JG,
Miller DA, et al. Spontaneous vaginal delivery: a risk factor
for Erb palsy? Am J Obstet Gynecol. 1998;178:423-427.
Gherman RB. Persistent
brachial plexus injury: The outcome of concern among patients
with
suspected fetal macrosomia. Am J Obstet Gynecol. 1998;178:195.
Gherman RB. Increased
cesarean delivery: No effect on brachial plexus palsy. Am J
Obstet
Gynecol. 1998;179:1378-1379.
Gherman RB, Ouzounia JG,
Goodwin TM. Brachial plexus palsy: An in utero injury? AM J
Obstet Gynecol. 1999;180:1303-1307.
Gherman RB, Tramont J,
Muffley P, Goodwin TM. Analysis of McRoberts' Maneuver by
X-ray
pelvimetry. Obstet Gynecol 2000;95:43-7.
Gherman RB. Fetal abdominal
circumference and macrosomia. J Reprod Med. 2001;46:699-700.
Gherman RB. Shoulder dystocia:
An evidence-based evaluation of the obstetrical nightmare.
Clinical Obstet and Gynecol 2002;45:345-361.
Gilbert A, Brockman R,
Carlioz H. Surgical treatment of brachial plexus birth palsy.
Clinical Orthopedics and Related Research, 1991;264:39-47.
Gilbert WM, Newbitt TS,
Danielsen B. Associated factors in 1611 cases of brachial
plexus
injury. Obstet Gynecol 1999;93:536-540.
Gilby JR, Williams MC,
Spellacy WN. Fetal abdominal circumference measurements of 35
and 38
cms as predictors of macrosomia: A risk factor for shoulder
dystocia. J Reprod Med.
2000;45:936-938.
Ginsberg NA, Moisidis C. How
to predict recurrent shoulder dystocia. Am J Obstet Gynecol.
2001;184:1427- 1430.
Golditch IM, Kirkman K. The
large fetus: management and outcome. Obstet Gynecol. 1978;52:
26-31.
Gonen R, Spiegel D, Abend M.
Is macrosomia predictable, and are shoulder dystocia and birth
trauma preventable? Obstet Gynecol. 1996;88:526-529.
Gonen O, Rosen DJ, Dolfin Z,
et al. Induction of labor versus expectant management in
macrosomia: A randomized study. Obstet Gynecol.
1997;89:913-917.
Gonen R, Bader D, Ajami M.
Effects of a policy of elective cesarean delivery in cases of
suspected fetal macrosomia on the incidence of brachial plexus
injury and the rate of
cesarean delivery. Am J Obstet Gynecol 2000;183:1296-1300.
Gonik B, Stringer CA, Held B.
An alternate mechanism for management of shoulder dystocia.
AM J Obstet Gynecol. 1983;145:882-884.
Gonik B, Allen R, Sorab J.
Objective evaluation of the shoulder dystocia phenomenon:
Effect of maternal pelvic orientation on force reduction.
Obstet Gynecol. 1989;74:44-47.
Gonik B, Hollyer VL, Allen R.
Shoulder dystocia recognition: Differences in neonatal risks
for injury. Am J Perinatol 1991;8:31-34.
Gonik B, Walker A, Grimm M.
Mathematic modeling of forces associated with shoulder
dystocia: A comparison of endogenous and exogenous sources. Am
J Obstet Gynecol.
2000;182;689-691.
Goodwin TM, Banks E, Millar
LK, Phelan JP. Catastrophic shoulder dystocia and emergency
symphysiotomy. Am J Obstet Gynecol 1997;177:463-4.
Gordon M, Rich H,
Deutschberger J, et al. The immediate and long-term outcome of
obstetric
birth trauma: I. Brachial plexus paralysis. Am J Obstet
Gynecol. 1973;117:51-56.
Graham EM, Forouzan I, Morgan
MA. A retrospective analysis of Erb palsy cases and their
relation to birth weight and trauma at delivery. J
Maternal-Fetal Medicine 1997;6:1-5.
Gregory KD, Henry OA,
Ramicone E, Chan LS, Platt LD. Maternal and infant
complications in
high and normal weight infants by method of delivery. Obstet
Gynecol 1998;92:507-13.
Gross SJ, Shime J, Farine D.
Shoulder dystocia: Predictors and outcome--a five year
review. Am J Obstet Gynecol 1987;156:334-336.
Gross TL, Sokol RJ, WIlliams
T, et al. Shoulder dystocia: a fetal-physician risk. Am J
Obstet Gynecol. 1987;156:1408-1418.
Gudmundsson S, Henningsson AC, Lindqvist P. Correlation of birth injury with maternal height and birthweight. BJOG. 2005 Jun;112(6):764-7
Hadlock, FP, Harrist, RB,
Sharman RS, Deter RL, Park SK. Estimation of fetal weight with
the use of head, body, and femur measurements--A prospective
study. Am J Obstet Gynecol
1985;151:333-7.
Hankins GDV, Clark SL.
Brachial plexus palsy involving the posterior shoulder at
spontaneous vaginal delivery. Am J Perinatology 1995;12:44-45.
Hankins GDV. Lower thoracic
spinal cord injury: A severe complication of shoulder
dystocia. Am J Perinatol. 1998;15:443-444.
Harris BA. Shoulder dystocia.
Clinical Ob Gyn 1984;27:106-111.
Hartfield VJ. Subcutaneous symphysiotomy -- time for a
reappraisal? Aust. N.Z.J. Obstet.
Gynaec. 1973;13:147-152.
Hartfield VJ. Letter to
editors: Symphysiotomy for shoulder dystocia. Am J Obstet
Gynecol
1986;228.
Hassan AA. Shoulder dystocia:
risk factors and prevention. Aust NZ J Obstet Gynaecol
1988;28:107-109.
Heath T, Gherman RB.
Symphyseal separation, sacro-iliac joint dislocation, and
transient
lateral femoral cutaneous neuropathy associated with McRoberts'
maneuver. J Reprod Med.
1999;44:902-904.
Hernandez C, Wendel GD.
Shoulder dystocia. Clinical Obstet and Gynecol
1990;33:526-534.
Hope P, Breslin S, Lamont L,
et al. Fatal shoulder dystocia: A review of 56 cases reported
to the Confidential Enquiry into Stillbirths and Deaths in
Infancy. Br J Obstet Gynecol.
1998;105:1256-1261.
Hopewood HG. Shoulder
dystocia: Fifteen years' experience in a community hospital.
Am J
Obstet. Gynecol 1982;144:162-166.
Iff L, Appuzzio J, Ganesh V.
Ltr re: A randomized controlled trial of prophylactic
maneuvers to reduce head-to-body delivery time in patients at
risk for shoulder dystocia.
Obstet Gynecol 2003;102:1089-90.
Jackson ST, Hoffer MM,
Parrish N. Brachial plexus palsy in the newborn. J Bone and
Joint
Surg 1988;70:1217- 1220.
Jazayeri A, Heffron JA,
Phillips R, et al. Macrosomia prediction using ultrasound
fetal
abdominal circumference of 35 centimeters or more. Obstet
Gynecol. 1999;93:523-526.
Jennett RJ, Tarby TJ,
Kreinick CJ. Brachial plexus palsy: An old problem revisited.
Am J
Obstet Gynecol 1992;166:1673-7.
Jennett RJ, Tarby TJ.
Brachial plexus palsy: An old problem revisited again. Am J
Obstet
Gynecol 1997;176:1354-1357.
Johar R, Rayburn W, Weir D,
Eggert L. Birth weights in term infants: A 50 year
perspective. J Repro Med 1988;33:813-816.
Johnson J, Longmate J,
Frentzen B. Excessive maternal weight and pregnancy outcome.
Am J
Obstet Gynecol. 1992;167:353.
Johnson NR. Shoulder dystocia:
A study of 47 cases. Aust N.Z.J. Obstet Gynaec
1979;19:28-31.
Kees S, Margalit V, Schiff E,
et al. Features of shoulder dystocia in a busy obstetric
unit. J Reprod Med. 2001;46:583-588.
Keller JD, Lopez-Zeno JA,
Dooley SL, et al. Shoulder dystocia and birth trauma in
gestational diabetes; A five-year experience. Am J Obstet
Gynecol. 1991;165:928-930.
Kinch RAH. Shoulder girdle
dystocia. Clin Obstet Gynecol. 1962;5:1031-1043.
Kitzmiller JL, Mall JC, Gin,
FD, Hendirkcs SK, Newman RB, Scheerer L. Measurement of fetal
shoulder width with computed tomography in diabetic women.
Obstet Gynecol 1987;70:941-945.
Klebanoff MA, Mills JL,
Berendes HW. Mothers birth weight as a predictor of macrosomia.
Am
J Obstet Gynecol. 1985; 153:253-258.
Koenigsberger MR. Brachial
plexus palsy at birth: Intrauterine or due to delivery trauma?
Annals of Neurology 1980;8:228.
Kolderup LB, Laros, Jr RK,
Musci TJ. Incidence of persistent birth injury in macrosomic
infants: Association with mode of delivery. Am J Obstet.
Gynecol 1997;177:37-41.
Langer O, Berkus MD, Huff RW,
Samueloff A. Shoulder dystocia: Should the fetus weighing
>/=4000gm be delivered by cesarean section? Am J Obstet
Gynecol 1991;165:831-7.
Langhoff-Roos J, Lindmark G,
Gebre-Medhin M. Maternal fat stores and fat accretion during
pregnancy in relation to infant birthweight. Brit J Obstet
Gynecol 1987;94:1170-1177.
Lazer S, Biale Yigal, Mazor
Mosme, Lewenthat H, Insler V. Complications associated with
the
macrosomic fetus. J Repro Med 1986;31:501-505.
Leaphart WL, Meyer MC,
Capeless EL. Labor induction with a prenatal diagnosis of
fetal
macrosomia. J Maternal Fetal Med. 1997;6:99-102.
Lee C. Shoulder Dystocia.
Clin Obstet Gynecol. 1978;30: 77-82.
Levine A, Lockwood CJ, Brown
B, Lapinski R, Berkowitz RL. Sonographic diagnosis of the
large for gestational age fetus at term: Does it make a
difference? Obstet Gynecol
1992;79:55-8.
Levine MG, Holroyde J, Woods
JR, Siddiqu TA, Scott M, Miodovnik M. Birth trauma: Incidence
and predisposing factors. Obstet Gynecol 1984;63:792.
Lewis DF, Raymond RC, Perkins
MB, Brooks GG, Heymann AR. Reucurrence rate of shoulder
dystocia. Am J Obstet Gynecol 1995;172:1369-71.
Lewis DF, Edwards MS, Asrat
T, et al. Can shoulder dystocia be predicted? Preconceptual
and prenatal factors. J Reprod Med. 1998;43:654-658.
Lipscomb KR, Gregory K, Shaw
K. The outcome of macrosomic infants weighing at least 4500
grams: Los Angeles County-University of Southern California
experience. Obstet Gynecol.
1995;85:558-564.
Lurie S, Levy R, Ben-Arie A,
Hagay Z. Shoulder dystocia: Could it be deduced from the
labor partogram? Am J Perinatology, 1995;12:61-62.
Lurie S, Insler V, Hagay Z.
Induction of labor at 38-39 weeks of gestation reduces the
incidence of shoulder dystocia in gestational diabetic
patients class A2. Am J
Perinatology 1996;13:293-296.
Mazouni C, Porcu G, Cohen-Solai E, Heckenroth H, Guidicelli B, Bonnier P, Gamerre M. Maternal and anthropomorphic risk factors for shoulder dystocia. Acta Obstet Gynecol Scand. 2006;85(5):567-70
McFarland LV, Raskin M,
Daling JR, Benedetti TJ. Erb/Duchenne's palsy: A consequence
of
fetal macrosomia and method of delivery. Obstet Gynecol
1986;68:784-8.
McFarland M, Hod M, Piper JM,
Xenakis EM, Langer Oded. Are labor abnormalities more common
in shoulder dystocia? Am J Obstet Gynecol 1995;173:1211-14.
McFarland MB, Langer O, Piper
JM, Berkus MD. Perinatal outcome and the type and number of
maneuvers in shoulder dystocia. Internat J Gynecol Obstet
1996;55:219-224.
McFarland MB, Trylovich CG,
Langer O. Anthropometric differences in macrosomic infants of
diabetic and nondiabetic mothers. J Maternal Fetal Med.
1998;7:292-295.
Menticoglou, SM.
Symphysiotomy for the trapped aftercoming parts of the breech:
A review
of the literature and a plea for its use. Aust & NZ J of
Obstet & Gynecol 1990;30:1-9.
Mintz MC, Landon MB, Gabbe SG,
Marinelli DL, Ludmir J, Grumbach K, Arger PH, Colemen BG.
Shoulder soft tissue width as a predictor of macrosomia in
diabetic pregnancies. Am J
Perinatology 1989;6:240-243.
Modanlou HD, Dorchester WL,
Thorosia A, Freeman RK. Macrosomia--maternal, fetal, and
neonatal implications. Obstet Gynecol 1980;55:420-424.
Modanlou HD, Komatsu G,
Dorchester W, Freeman RK, Bosv SK. Large-for-gestational -age
neonates: Antropometric reasons for shoulder dystocia. Obstet
Gynecol 1982;60:417-23.
Morris WIC. Shoulder dystocia.
J. Obstet Gynaecol Br Empire. 1955;62:302-306.
Morrison JC, Sanders JR,
Magann EF, Wiser WL. The diagnosis and management of dystocia
of
the shoulder. Surg Gynecol Obstet 1992;175:515-22.
Nasrat H, Abalkail B, Fageeh
W, et al. Anthropometric measurements of newborns of
gestational diabetic mothers: Does it indicate disproportional
fetal growth? J Maternal
Fetal Med. 1997;6:291-295
Nesbitt TS, Gilbert WM,
Herrchen B. Shoulder dystocia and associated risk factors with
macrosomic infants born in California. Am J Obstet Gynecol.
1998;179:476-480.
Nocon JJ, McKenzie DK, Thomas
LJ, Hansell RS. Shoulder dystocia: An analysis of risks and
obstetric maneuvers. Am J Obstet Gynecol 1993;168:1732-1739.
Nocon, James J., Les Weisbrod.
"Shoulder Dystocia" Chap 14 of Operative Obstetrics
Edited by John Patrick O'Grady and Martin Gimovsky. Williams
and Wilkins. 1995:339-353.
O'Leary JA, Leonetti HB.
Shoulder dystocia: Prevention and treatment. Am J Obstet
Gynecol
1990;162:5-9.
O'Leary, James. Shoulder
Dystocia and Birth Injury. McGraw-Hill, Inc. New York: 1992.
O'Leary JA, Cuva A. Abdominal
rescue after failed cephalic replacement. Obstet Gynecol.
1992;80:514-516.
O'Leary JA. Cephalic
replacement for shoulder dystocia: Present status and future
role of
the Zavanelli maneuver. Obstet Gynecol 1993;82:847-50.
Oppenheim WL, Davis A,
Growdon WA, Dorey FJ, Davlin LB. Clavicle fractures in the
newborn.
Clinical Orthopedics and Related Research, 1990;#250:176-180.
O'Shaughnessy MJ. Hysterotomy
facilitation of the vaginal delivery of the posterior arm in
a case of shoulder dystocia. Obstet Gynecol. 1998;92:693-695.
Ouzounian JG, Korst LM,
Phelan JP. Permanent Erb palsy: A traction-related injury?
Obstet
Gynecol 1997;89:139-141.
Ouzounian JG, Korst LM, Ahn
MO, et al. Shoulder dystocia and neonatal brain injury:
Significance of the head-shoulder interval. Am J Obstet
Gynecol. 1998;178:S76.
Parks DG, Ziel HK. Macrosomia,
a proposed indication for primary cesarean section. Obstet
Gynecol. 1978;52:407-412.
Pecorari D. A guest editorial
from abroad: Meditations on a nightmare of modern midwifery:
Shoulder dystocia. Obstet Gynecol Surv. 1999:54:353-354.
Pecorari D. A guest
editorial: Erb palsy without apparent shoulder dystocia.
Obstet
Gynecol Survey 2002;9:547.
Peleg D, Hasnin J, Shalev E.
Fractured clavicle and Erb palsy unrelated to birth trauma.
Am J Obstet Gynecol 1997;177:1038040.
Phelan JP, Ouzounian JG,
Gherman RB, et al. Shoulder dystocia and permanent Erb palsy:
the
role of fundal pressure. Am J Obstet Gynecol. 1997;176:S138.
Philpot J, Muntoni F,
Skellett S, Dubowitz V. Congenital symmetrical weakness of the
upper
limbs resembling brachial plexus palsy: A possible sequel of
drug toxicity in first
trimester of pregnancy? Neuromusc. Disord. 1995;5:67-69.
Ramsey PS, Ramin KD, Field
CS. Shoulder dystocia: Rotational maneuvers revisited. J
Reprod Med. 2000;45:85-88.
Resnik R, Management of
shoulder girdle dystocia. Clinical Obstetrics and Gynecology
1980;23:559-564.
Roberts SW, Hernandez C,
Maaberry MC, Adams MD, Leveno KJ, Wendel GD. Obstetric
clavicular
fracture: The enigma of normal birth. Obstet Gynecol
1995;86:978-81.
Rossavik IK, Joslin GL.
Macrosomatia and ultrasonography: What is the problem?
Southern
Medical Journal 1993;86:1129-1132.
Rouse DJ, Owen J, Goldenberg
RL, Cliver SP. The effectiveness and costs of elective
cesarean delivery for fetal macrosomia diagnosed by
ultrasound. JAMA 1996;276:1480-6.
Rouse DJ, Owen J.
Prophylactic cesarean delivery for fetal macrosomia diagnosed
by means of
ultrasonography: A Faustian bargain? Am J Obstet Gynecol.
1999;81:332-338.
Russell JGB. The rationale of
primitive delivery positions. British J of Obstet and
Gynaecol 1982;89:712-5.
Sack RA. The large infant: a
study of maternal, obstetric, fetal, and newborn
characteristics including a long-term pediatric follow-up. Am
J Obstet Gynecol. 1969; 104:
195-204.
Sacks DA, Chen W. Estimating
fetal weight in the management of macrosomia. Obstet Gynecol
Surv. 2000;55:229-239.
Sanberg EC. The Zavanelli
maneuver: 12 years of recorded experience. Obstet Gynecol.
1999;93:312-317.
Sandmire HF, O'Halloin TJ,
Shoulder dystocia: Its incidence and associated risk factors.
Int J Gynaecol Obstet. 1988;26:65-73.
Sandmire HF. Whither
ultrasonic prediction of fetal macrosomia? Obstet Gynecol.
1993;82:860-862.
Sandmire HF, DeMott RK. The
Green Bay cesarean section study: IV. The physician factor as
a determinant of cesarean birth rates for the large fetus. Am
J Obstet Gynecol
1996;174:1557-1564.
Sandmire HF, DeMott RK. Erb
palsy: Concepts of causation. Obstet Gynecol.
2000;95:940-942.
Sarno Maj AP, Hinderstein LTC
WN, Staiano, Col RA. Fetal macrosomia in a military hospital:
Incidence, risk factors , and outcome. Military Medicine
1991;156:55-58.
Schramm M. Impacted
shoulders: A personal experience. Aust NZ J Obstet Gyneacol.
1983;23:28-31.
Sherman DJ, Arieli S, Tovbin
J, Siegel G, Caspi E, Bukovsky I. A comparison of clinical and
ultrasonic estimation of fetal weight. 1998;91:212-217.
Smith RB, Lane C, Pearson JF.
Shoulder dystocia: What happens at the next delivery? Br J
Obstet Gynecol. 1994;101:713-715.
Skolbekken JA. Shoulder
dystocia: malpractice or acceptable risk? Acta Obstet Gynecol
Scand. 2000;79:750-756.
Sorab J, Allen RH, Gonik B.
Tactile sensory monitoring of clinician-applied forces during
delivery of newborns. IEEE Transactions on Biomedical
Engineering 1988;35:1090-1093.
Spellacy WN, Miller MS,
Winegar A, Peterson PQ. Macrosomia -- maternal characteristics
and
infant complications. Obstetrics Gynecology. 1985; 66:158-162.
Spong CY, Beall M, Rodrigues
D, Ross MG. An objective definition of shoulder dystocia:
Prolonged head-to-body delivery intervals and/or the use of
ancillary obstetric maneuvers.
Obstet Gynecol 1995;86:433-6.
Stallings SP, Edwards RK,
Johnson JWC. Correlation of head-to-body delivery intervals in
shoulder dystocia and umbilical artery acidosis. Am J Obstet
Gynecol. 2001;185:268-274.
Swartz DP. Shoulder girdle
dystocia in vertex delivery: clinical study and review. Obstet
Gynecol. 1960;15:194-206.
Tamura RK, Sabbagha RE, Depp
R, Dooley SL, Socol ML. Diabetic macrosomia: Accuracy of
third trimester ultrasound. Obstet Gynecol 1986;67:828-832.
Turnpenny RD. Fractured
clavicle of the newborn in a population with a high prevalence
of
grand-multiparity: analysis of 78 consecutive cases. British J
of Obstet and Gynaecology
1993;100:338-341.
Warsof SL, Gohari P,
Berkowitz RL, Hobbins JC. The estimation of fetal weight by
computer-assisted analysis. Am J Obstet. Gynecol
1977;128:881-892.
Widness JA, Schwartz HC,
Thompson D, Kahn CB, Oh W, Schwartz R. Hemoglobin A1c
(glycohaemoglobin) in diabetic pregnancy: An indicator of
glucose control and fetal size.
British J of Obstet & Gynaecol 1978A;85:812-17.
Widness JA, Schwartz HC,
Thompson D, King KC, Kahn CB, Oh W, Schwartz R.
Glycohemoglobin
(HbA1c): A predictor of birth weight in infants of diabetic
mothers. J Pediatrics
1978B;92:8-12.
Williams Obstetrics, 21st
Edition. F. Gary Cunningham, ed. McGraw-Hill. 2001.
Wladimiroff JW, Bloemsam CA,
Wallenburg HCS. Ultrasonic diagnosis of the large-for-dates
infant. Obstet Gynecol 1978;52:285-288.
Wood C, Ng KH, Hounslaw D, et
al. Time: an important variable in normal delivery. J
Obstet Gynaecol Br Commonw. 1973;80:295-300.
Zisow DL. Uterine rupture as
a cause of shoulder dystocia. Obstet Gynecol.
1996;87:818-819.
Copyright ©
2017 Henry Lerner